Abstract
Purpose
Peripheral neuropathy (PN) is an intractable side effect of oxaliplatin, with no effective prophylaxis so far. Nin**’yoeito (NYT), a Kampo medicine, is protective against oxaliplatin-induced neuronal cell injury in vitro and ameliorates oxaliplatin-induced PN in vivo. Thus, this randomized controlled trial was aimed at clarifying NYT’s prophylactic effect for oxaliplatin-induced cumulative PN.
Methods
52 patients with colorectal cancers of pathological stage 3 received postoperative adjuvant chemotherapy with the CapeOX regimen: eight cycles of capecitabine (2400 mg/m2) plus oxaliplatin (130 mg/m2) at 3-week intervals. They were randomly assigned to NYT administration and non-administration groups. NYT (9.0 g/day) was administered from day 1 of cycle 1 in the NYT group. The NYT was administered orally daily throughout each cycle. The primary endpoint was the grade of cumulative PN at the end of eight cycles. The secondary endpoints included relative dose intensity (RDI) of oxaliplatin, recurrence-free survival (RFS), and overall survival (OS).
Results
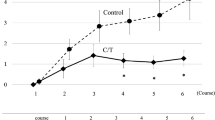
40 patients (n = 20 in both groups) completed 8 chemotherapy cycles. The incidence of grade 2 or greater cumulative PN at the 8th chemotherapy cycle was significantly lower in the NYT group (2/20, 10.0%) than in the control group (11/20, 55.0%, P < 0.01). RDI of oxaliplatin was significantly higher in the NYT group than in the control group (P = 0.02). RFS and OS were better in the NYT group than in the control group, but the difference was not significant.
Conclusions
NYT may reduce the incidence of oxaliplatin-induced cumulative PN and facilitate maintenance of the CapeOX dosing regimen.




Similar content being viewed by others
References
Griffith KA, Zhu S, Johantgen M et al (2017) Oxaliplatin-induced peripheral neuropathy and identification of unique severity groups in colorectal cancer. J Pain Symptom Manage 54:701–706
Nishina T, Kato T, Yamazaki K et al (2015) A phase II study of S-1, oxaliplatin, oral leucovorin, and bevacizumab combination therapy (SOLA) in patients with unresectable metastatic colorectal cancer. Cancer Chemother Pharmacol 76:547–553
Motoo Y, Seki T, Tsutani K (2011) Traditional Japanese medicine, Kampo: its history and current status. Chin J Integr Med 17:85–87
Motoo Y, Hakamatsuka T, Kawahara N et al (2017) Standards of Reporting Kampo Products (STORK) in research articles. J Integr Med 15:182–185
Nishioka M, Shimada M, Kurita N et al (2011) The Kampo medicine, Gosha**kigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol 16:322–327
Kono T, Hata T, Morita S et al (2013) Gosha**kigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of gosha**kigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol 72:1283–1290
Oki E, Emi Y, Kojima H et al (2015) Preventive effect of Gosha**kigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol 20:767–775
Hoshino N, Ganeko R, Hida K et al (2018) Gosha**kigan for reducing chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Int J Clin Oncol 23:434–442
Kuriyama A, Endo K (2018) Gosha**kigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer 26:1051–1059
Motoo Y, Mouri H, Ohtsubo K et al (2005) Herbal medicine Nin**yoeito ameliorates ribavirin-induced anemia in chronic hepatitis C: a randomized controlled trial. World J Gastroenterol 11:4013–4017
Miyano K, Nonaka M, Uzu M et al (2018) (2018) Multifunctional actions of nin**yoeito, a Japanese Kampo medicine: accumulated scientific evidence based on experiments with cells and animal models, and clinical studies. Front Nutr 5:93. https://doi.org/10.3389/fnut.2018.00093.eCollection
Suzuki T, Yamamoto A, Ohsawa M et al (2015) Nin**'yoeito and ginseng extract prevent oxaliplatin-induced neurodegeneration in PC12 cells. J Nat Med 69:531–537
Suzuki T, Yamamoto A, Ohsawa M et al (2017) Effect of nin**'yoeito and ginseng extracts on oxaliplatin-induced neuropathies in mice. J Nat Med 71:757–764
Danno K, Hata T, Tamai K et al (2017) Interim analysis of a phase II trial evaluating the safety and efficacy of capecitabine plus oxaliplatin (XELOX) as adjuvant therapy in Japanese patients with operated stage III colon cancer. Cancer Chemother Pharmacol 80:777–785
Mandrekar SJ, Sargent DJ (2010) Randomized phase II trials: time for a new era in clinical trial design. J Thorac Oncol 5:932–934
Schmoll HJ, Tabernero J, Maroun J et al (2015) Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 33(32):3733–3740
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Goldberg RM, Gill S (2004) Recent phase III trials of fluorouracil, irinotecan, and oxaliplatin as chemotherapy for metastatic colorectal cancer. Cancer Chemother Pharmacol 54:S57–64
Kaku H, Kumagai S, Onoue H et al (2012) Objective evaluation of the alleviating effects of gosha**kigan on PN induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med 3:60–65
Abe H, Kawai Y, Mori T et al (2013) The Kampo medicine gosha**kigan prevents neuropathy in breast cancer patients treated with docetaxel. Asian Pac J Cancer Prev 14:6351–6356
Seiwa C, Yamamoto M, Tanaka K et al (2007) Restoration of FcRgamma/Fyn signaling repairs central nervous system demyelination. J Neurosci Res 85:954–966
González-Burgos E, Fernandez-Moriano C, Gómez-Serranillos MP (2015) Potential neuroprotective activity of Ginseng in Parkinson's disease: a review. J Neuroimmune Pharmacol 10:14–29
Sato N, Seiwa C, Uruse M et al (2011) Administration of chinpi, a component of the herbal medicine nin**-youei-to, reverses age-induced demyelination. Evid Based Complement Alternat Med 2011:617438
Acknowledgements
This study was supported in part by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K09267).
Author information
Authors and Affiliations
Contributions
All the authors contributed to this study. YM designed this trial, enrolled the patients, and analyzed the data. YT and HF took care of the patients.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Motoo, Y., Tomita, Y. & Fujita, H. Prophylactic efficacy of nin**’yoeito for oxaliplatin-induced cumulative peripheral neuropathy in patients with colorectal cancer receiving postoperative adjuvant chemotherapy: a randomized, open-label, phase 2 trial (HOPE-2). Int J Clin Oncol 25, 1123–1129 (2020). https://doi.org/10.1007/s10147-020-01648-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01648-3