Abstract
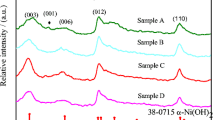
Samples of [Ni4Al(OH)10]OH were prepared by co-precipitation with the existence of calcium and subsequent hydrothermal treatment. Inductively coupled plasma (ICP) measurements show that the composition of prepared samples does not change very much with the initial concentration of Ca2+ in the mother solution, which may be related to the high solubility of Ca(OH)2. Powder X-ray diffraction measurements show that the modification does not change the lattice parameters of [Ni4Al(OH)10]OH a lot, but scanning electron microscope images show some morphological differences between the sample without Ca and the sample after the modification of calcium hydroxide. However, those changes in composition and morphology do improve the reversibility and charge efficiency of [Ni4Al(OH)10]OH, especially at a higher temperature of 65 °C. For example, the difference between the oxidation peak and oxygen evolution peak in the cyclic voltammetric diagrams at 65 °C becomes large, a better charge/discharge performance at high current density can be observed. In addition, the charge-transfer resistance (R t) of the electrode according to the electrochemical impedance spectra increases after the modification of calcium and become larger as the temperature is elevated for 20 to 65 °C.








Similar content being viewed by others
References
Ovshinsky SR, Fetcenko MA, Ross J (1993) Science 260:176
Linden D (1995) Handbook of batteries. McGraw-Hill, New York
Fetcenko MA, Ovshinsky SR, Reichman B, Young K, Fierro C, Koch J, Zallen A, Mays W, Ouchi T (2007) J Power Sources 165:544
Barnard R, Randell CF, Tye FL (1980) J Appl Electrochem 10:127
Corrigan DA (1989) J Electrochem Soc 136:7
Faure C, Delmas C, Willmann P (1991) J Power Sources 36:497
Hu WK, Noreus D (2003) Chem Mater 15:974
Hu M, Lei L (2007) J Solid State Electrochem 11:847
Lei L, Hu M, Gao X, Sun Y (2008) Electrochimica Acta 54:671
Hu M, Gao X, Lei L, Sun Y (2009) J Phys Chem C 113:7448
Guerlou-Demourgues L, Fournes L, Delmas C (1996) J Electrochem Soc 143:3083
Wu MY, Wang JM, Zhang JQ, Cao CN (2006) J Solid State Electrochem 10:411
Chen H, Wang JM, Zhao YL, Zhang JQ, Cao CN (2005) J Solid State Electrochem 9:421
Kamath PV, Dixit M, Indira L, Shukla AK, Kumar VG, Munichandraiah N (1994) J Electrochem Soc 141:2956
Wang CY, Zhong S, Bradhurst DH, Liu HK, Dou SX (2002) J Alloy Compd 330–332:802
Gao X, Lei L, Hu M, Qin L, Sun Y (2009) J Power Sources 191:662
Shinyama K, Magari Y, Funahashi A, Tanaka K (2003) Electrochemistry 71:686
Hu WK, Gao XP, Geng MM, Gong ZX, Noreus D (2005) J Phys Chem B 109:5392
Watanabe K, Koseki M, Kumagai N (1996) J Power Sources 58:23
Pralong V, Delahaye-Vidal A, Beaudoin B, Leriche JB, Tarascon JM (2000) J Electrochem Soc 147:1306
Tessier C, Faure C, Guerlou-Demourgues L, Denage C, Nabias G, Delmas C (2002) J Electrochem Soc 149:A1136
Oshitani M, Watada M, Shodai K, Kodama M (2001) J Electrochem Soc 148:A67
Nan J, Hou X, Yang M, Han D, Li W (2006) J Electrochem Soc 153:A1159
Yuan AB, Cheng SO, Zhang JQ, Cao CN (1998) J Power Sources 76:36
He XM, Ren JG, Li W, Jiang CY, Wan CR (2006) Electrochim Acta 51:4533
Zhang X, Gong Z, Zhao S, Geng M, Wang Y, Northwood DO (2008) J Power Sources 175:630
Begum SN, Muralidharan VS, Ahmed Basha C (2009) Int J Hydrogen Energy 34:1548
Kibria MF, Mridha MS (1996) Int J Hydrogen Energy 21:179
Unates ME, Folquer ME, Vilche JR, Arvia AJ (1992) J Electrochem Soc 139:2697
Maeda A, Kimiya H, Moriwaki Y, Matsumoto I, Maita A, Kimiya K (1999) EP923146A1
Liu B, Yuan H, Zhang Y (2004) Int J Hydrogen Energy 29:453
Zhao YL, Wang JM, Chen H, Pan T, Zhang JQ, Cao CN (2004) Electrochim Acta 50:91
Brug GJ, van den Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) J Electroanal Chem 176:275
Rammelt U, Reinhard G (1990) Electrochimica Acta 35:1045
Viswanathan VV, Salkind AJ, Kelley JJ, Ockerman JB (1995) J Appl Electrochem 25:716
Reid MA, Loyselle PL (1991) J Power Sources 36:285
Wang XY, Yan J, Yuan HT, Zhang YS, Song DY (1999) Int J Hydrogen Energy 24:973
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, L., Hu, M., Gao, X. et al. Effect of calcium hydroxide on the electrochemical performance of a [Ni4Al(OH)10]OH electrode. J Solid State Electrochem 15, 405–412 (2011). https://doi.org/10.1007/s10008-010-1102-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1102-0