Abstract
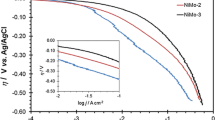
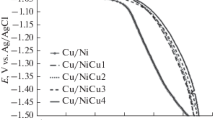
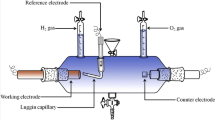
In this paper, NiMo coatings were electrochemically deposited on a copper electrode (Cu/NiMo) and on an electrodeposited nickel onto copper plate (Cu/Ni/NiMo) in citrate solutions. Effects of electrolyte composition, pH value, and temperature on hydrogen-evolution reaction (HER) as well as the electrochemical stability in alkaline solution were investigated, and the electrochemical activation energy was determined for the NiMo alloys. This was evaluated by the determination of kinetic and mechanism of HER in alkali medium by the polarization measurements, cyclic voltammetry, and electrochemical impedance spectroscopy techniques. The morphology and chemical composition of the electrodeposited Ni–Mo were investigated using SEM and EDS analyses. The results showed that the corresponding HER overpotential of the Ni–Mo film depends on alloy composition and surface morphology. As the wt% of Mo content in the alloy is increased, the onset potential of electrode for HER shifted in the positive direction favoring hydrogen generation with lower overpotential. The overall experimental data indicated that the porous Ni–Mo coating on electrodeposited nickel plate was obtained when the molybdenum content was ca. 41 wt%. This electrodes exhibited high catalytic activity in the HER (η 100 = −48 mV at 100 mA cm−2 and 80 °C), and their stability was tested by polarization measurements after different anodic and cathodic treatments in 1 M NaOH solution. Moreover, the corrosion behaviors of Ni and Cu/Ni/NiMo electrodes at open-circuit potential were also investigated, and their corrosion resistances were compared.
Graphical abstract









Similar content being viewed by others
References
Veziroğlu TN, Barbir F (1992) Hydrogen: the wonder fuel. Int J Hydrogen Energy 17:391–404. doi:10.1016/0360-3199(92)90183-W
Veziroğlu TN, Sxahin S (2008) 21st Century’s energy hydrogen energy system. Energy Convers Manag 49(7):1820–1831. doi:10.1016/j.enconman.2007.08.015
Döner A, Taşkesen E, Kardaş G (2014) Hydrogen evolution stability of platinum modified graphite electrode. Int J Hydrogen Energy 39:11355–11359. doi:10.1016/j.ijhydene.2014.05.159
Solmaz R, Kardaş G (2011) Fabrication and characterization of NiCoZn–M (M: Ag, Pd and Pt) electrocatalysts as cathode materials for electrochemical hydrogen production. Int J Hydrogen Energy 36:12079–12087. doi:10.1016/j.ijhydene.2011.06.101
Pletcher D, Li X (2011) Prospects for alkaline zero gap water electrolysers for hydrogen production. Int J Hydrogen Energy 36:15098–15104. doi:10.1016/j.ijhydene.2011.08.080
McArthur MA, Jorge L, Coulombe S, Omanovic S (2014) Synthesis and characterization of 3D Ni nanoparticle/carbon nanotube cathodes for hydrogen evolution in alkaline electrolyte. J Power Sour 266:365–373. doi:10.1016/j.jpowsour.2014.05.036
Rami A, Lasia A (1992) Kinetics of hydrogen evolution on Ni-Al alloy electrodes. J Appl Electrochem 22:376–382. doi:10.1007/BF01092692
Suffredini HB, Cerne JL, Crnkovic FC, Machado SAS, Avaca LA (2000) Recent developments in electrode materials for water electrolysis. Int J Hydrogen Energy 25:415–423. doi:10.1016/S0360-3199(99)00049-X
Tang X, **ao L, Yang C, Lu J, Zhuang L (2014) Noble fabrication of Ni-Mo cathode for alkaline water electrolysis and alkaline polymer electrolyte water electrolysis. Int J Hydrogen Energy 39:3055–3060. doi:10.1016/j.ijhydene.2013.12.053
Raj IA, Vasu K (1990) Transition metal-based hydrogen electrodes in alkaline solution-electrocatalysis on nickel based binary alloy coatings. J Appl Electrochem 20:32–38. doi:10.1007/BF01012468
Pletcher D, Li X, Wang S (2012) A comparison of cathodes for zero gap alkaline water electrolysers for hydrogen production. Int J Hydrogen Energy 37:7429–7435. doi:10.1016/j.ijhydene.2012.02.013
Hu C-C, Weng C-Y (2000) Hydrogen evolving activity on nickel–molybdenum deposits using experimental strategies. J Appl Electrochem 30:499–506. doi:10.1023/A:1003964728030
Donten M, Cesiulis H, Stojek Z (2005) Electrodeposition of amorphous/nanocrystalline and polycrystalline Ni–Mo alloys from pyrophosphate baths. Electrochim Acta 50:1405–1412. doi:10.1016/j.electacta.2004.08.028
Chassaing E, Portail N, Levy AF, Wang G (2004) Characterisation of electrodeposited nanocrystalline Ni–Mo alloys. J Appl Electrochem 34:1085–1091. doi:10.1007/s10800-004-2460-z
Sanches LS, Domingues SH, Marino CEB, Mascaro LH (2004) Characterisation of electrochemically deposited Ni–Mo alloy coatings. Electrochem Commun 6:543–548. doi:10.1016/j.elecom.2004.04.002
Crousier J, Eyraud M, Crousier JP, Roman JM (1992) Influence of substrate on the electrodeposition of nickel-molybdenum alloys. J Appl Electrochem 22:749–755. doi:10.1007/BF01027505
Sanches LS, Marino CB, Mascaro LH (2007) Investigation of the codeposition of Fe and Mo from sulphate-citrate acid solutions. J Alloy Compd 439:342–345. doi:10.1016/j.jallcom.2006.08.231
Marlot A, Kern P, Landolt D (2002) Pulse plating of Ni–Mo alloys from Ni–rich electrolytes. Electrochim Acta 48:29–36. doi:10.1016/S0013-4686(02)00544-3
Jović BM, Jović VD, Maksimović VM, Pavlović MG (2008) Characterization of electrodeposited powders of the system Ni–Mo–O. Electrochim Acta 53:4796–4804. doi:10.1016/j.electacta.2008.02.004
Han Q, Cui S, Pu N, Chen J, Liu K, Wei X (2010) A study on pulse plating amorphous Ni–Mo alloy coating used as HER cathode in alkaline medium. Int J Hydrogen Energy 35:5194–5201. doi:10.1016/j.ijhydene.2010.03.093
Krstajic NV, Jovic VD, Lj Gajic-Krstaji, Jovic BM, Antozzi AL, Martelli GN (2008) Electrodeposition of Ni–Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution. Int J Hydrogen Energy 33:3676–3687. doi:10.1016/j.ijhydene.2008.04.039
Aaboub O (2011) Hydrogen evolution activity of Ni–Mo coating electrodeposited under magnetic field control. Int J Hydrogen Energy 36:4702–4709. doi:10.1016/j.ijhydene.2011.01.035
Krstajić NV, Lj Gajić-Krstajić, Lačnjevac U, Jović BM, Mora S, Jović VD (2011) Non-noble metal composite cathodes for hydrogen evolution. Part I: the Ni–MoOx coatings electrodeposited from Watt’s type bath containing MoO3 powder particles. Int J Hydrogen Energy 36:6441–6449. doi:10.1016/j.ijhydene.2011.02.105
Krstajić NV, Lačnjevac U, Jović BM, Mora S, Jović VD (2011) Non-noble metal composite cathodes for hydrogen evolution. Part II: the Ni–MoO2 coatings electrodeposited from nickel chloride-ammonium chloride bath containing MoO2 powder particles. Int J Hydrogen Energy 36:6450–6461. doi:10.1016/j.ijhydene.2011.02.106
**a M, Lei T, Lv N, Li N (2014) Synthesis and electrocatalytic hydrogen evolution performance of Ni–Mo–Cu alloy coating electrode. Int J Hydrogen Energy 39:4797–4802. doi:10.1016/j.ijhydene.2014.01.091
Gennero de Chialvo MR, Chialvo AC (1998) Hydrogen evolution reaction on smooth Ni(1−x) + Mo(x) alloys (0 ≤ x ≤ 0.25). J Electroanal Chem 448:87–93. doi:10.1016/S0022-0728(98)00011-4
Beltowska-Lehman E, Indyka P (2012) Kinetics of Ni–Mo electrodeposition from Ni-rich citrate baths. Thin Solid Films 520:2046–2051. doi:10.1016/j.tsf.2011.10.024
Jaksic JM, Vojnovic MV, Krstajic NV (2000) Kinetic analysis of hydrogen evolution at Ni–Mo alloy electrodes. Electrochim Acta 45:4151–4158. doi:10.1016/S0013-4686(00)00549-1
Kaninski MPM, Miulovic SM, Tasic GS, Maksic AD, Nikolic VM (2011) A study on the Co–W activated Ni electrodes for the hydrogen production from alkaline water electrolysis—energy saving. Int J Hydrogen Energy 36:5227–5235. doi:10.1016/j.ijhydene.2011.02.046
Herraiz-Cardona I, Ortega E, Garcίa Antόn J, Pérez-Herranz V (2011) Assessment of the roughness factor effect and the intrinsic catalytic activity for hydrogen evolution reaction onNi-based electrodeposits. Int J Hydrogen Energy 36:9428–9438. doi:10.1016/j.ijhydene.2011.05.047
Los P, Rami A, Lasia A (1993) Hydrogen evolution reaction on Ni-Al electrodes. J Appl Electrochem 23:135–140
Highfield JG, Claude E, Oguro K (1999) Electrocatalytic synergism in Ni–Mo cathodes for hydrogen evolution in acid medium: a new model. Electrochim Acta 44:2805–2814. doi:10.1016/S0013-4686(98)00403-4
Damian A, Omanovic S (2006) Ni and Ni–Mo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J Power Sources 158:464–476. doi:10.1016/j.jpowsour.2005.09.007
Navvaro-Flores E, Chong Z, Omanovic S (2005) Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J Mol Catal A 226:179–197. doi:10.1016/j.molcata.2004.10.029
Jaksic MM (2000) Hypo–hyper–d–electronic interactive nature of synergism in catalysis and electrocatalysis for hydrogen reactions. Electrochim Acta 45:4085–4099. doi:10.1016/S0360-3199(00)00120-8
Eliaz N, Gileadi E (2007) The mechanism of induced codeposition of Ni–W alloys. Electrochem Society 6:337–349
Metzler OY, Zhu L, Gileadi E (2003) The anomalous codeposition of tungsten in the presence of nickel. Electrochim Acta 48:2551–2562. doi:10.1016/S0013-4686(03)00297-4
Eliaz N, Sridhara TM, Gileadi E (2005) Synthesis and characterization of nickel tungsten alloys by electrodeposition. Electrochim Acta 50:2893–2904. doi:10.1016/j.electacta.2004.11.038
Solmaz R, Kardaş G (2007) Hydrogen evolution and corrosion performance of NiZn coatings. Energy Convers Manag 48:583–591. doi:10.1016/j.enconman.2006.06.004
Conway BE, Jerkiewicz G (2000) Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the volcano curve for cathodic H2 evolution kinetics. Electrochim Acta 45:4075–4083. doi:10.1016/S0013-4686(00)00523-5
Krstajić N, Popović M, Grgur B, Vojnović M, Šepa D (2001) On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution Part I. The mechanism. J Electroanal Chem 512:16–26. doi:10.1016/S0022-0728(01)00590-3
Hu W (2000) Electrocatalytic properties of new electrocatalysts for hydrogen evolution in alkaline water electrolysis. Int J Hydrogen Energy 25:111–118. doi:10.1016/S0360-3199(99)00024-5
Metikos-Hukovic M, Jukic A (2000) Correlation of electronic structure and catalytic activity of Zr–Ni amorphous alloys for the hydrogen evolution reaction. Electrochim Acta 45:4159–4170. doi:10.1016/S0013-4686(00)00550-8
Elumalai P, Vasan HN, Munichandraiah N, Shivashankar SA (2002) Kinetics of hydrogen evolution on submicron size Co, Ni, Pd and Co–Ni alloy powder electrodes by d.c. polarization and a.c. impedance studies. J Appl Electrochem 32:1005–1010. doi:10.1023/A:1020935218149
Łosiewicz B, Budniok A, Rόwiński E, Łagiewka E, Lasia A (2004) The structure, morphology and electrochemical impedance study of the hydrogen evolution reaction on the modifed nickel electrodes. Int J Hydrogen Energy 29:145–157. doi:10.1016/S0360-3199(03)00096-X
Hitz C, Lasia A (2001) Experimental study and modeling of impedance of the her on porous Ni electrodes. J Electroanal Chem 500:213–222. doi:10.1016/S0022-0728(00)00317-X
Döner A, Solmaz R, Kardaş G (2011) Enhancement of hydrogen evolution at cobalt–zinc deposited graphite electrode in alkaline solution. Int J Hydrogen Energy 36:7391–7397. doi:10.1016/j.ijhydene.2011.03.083
Solmaz R, Kardaş G (2009) Electrochemical deposition and characterization of NiFe coatings aselectrocatalytic materials for alkaline water electrolysis. Electrochim Acta 54:3726–3734. doi:10.1016/j.electacta.2009.01.064
Birry L, Lasia A (2004) Studies of the hydrogen evolution reaction on Raney nickel–molybdenum electrodes. J Appl Electrochem 34:735–749
Kubisztal J, Budniok A, Lasia A (2007) Study of the hydrogen evolution reaction on nickel-based composite coatings containing molybdenum powder. Int J Hydrogen Energy 32:1211–1218. doi:10.1016/j.ijhydene.2006.11.020
Solmaz R, Döner A, Kardaş G (2008) Electrochemical deposition and characterization of NiCu coatings as cathode materials for hydrogen evolution reaction. Electrochem Commun 10:1909–1911. doi:10.1016/j.elecom.2008.10.011
Kawashima A, Sakaki T, Habazaki H, Hashimoto K (1999) Ni–Mo–O alloy cathodes for hydrogen evolution in hot concentrated NaOH solution. Mater Sci Eng, A 267:246–253. doi:10.1016/S0921-5093(99)00099-4
Niedbala J, Budniok A, Lagiewka E (2008) Hydrogen evolution on the polyethylene-modified Ni–Mo composite layers. Thin Sol Films 516:6191–6196. doi:10.1016/j.tsf.2007.11.105
Özkan S, Hapçı G, Orhan G, Kazmanlı K (2013) Electrodeposited Ni/SiC nanocomposite coatings and evaluation of wear and corrosion properties. Surf Coat Technol 232:734–741. doi:10.1016/j.surfcoat.2013.06.089
Acknowledgments
The authors gratefully acknowledge the financial support of the Scientific Research Projects Coordination Unit of Istanbul University (Project Number 22847).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manazoğlu, M., Hapçı, G. & Orhan, G. Effect of electrolysis parameters of Ni–Mo alloy on the electrocatalytic activity for hydrogen evaluation and their stability in alkali medium. J Appl Electrochem 46, 191–204 (2016). https://doi.org/10.1007/s10800-015-0908-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0908-y