Abstract
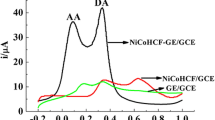
We report on a sensitive electrochemical sensor for dopamine (DA) based on a glassy carbon electrode that was modified with a nanocomposite containing electrochemically reduced graphene oxide (RGO) and palladium nanoparticles (Pd-NPs). The composite was characterized by scanning electron microscopy, energy dispersive spectroscopy, and electrochemical impendence spectroscopy. The electrode can oxidize DA at lower potential (234 mV vs Ag/AgCl) than electrodes modified with RGO or Pd-NPs only. The response of the sensor to DA is linear in the 1–150 μM concentration range, and the detection limit is 0.233 μM. The sensor was applied to the determination of DA in commercial DA injection solutions.

Schematic representation showing the oxidation of DA at RGO-Pd-NPs composite electrode.





Similar content being viewed by others
References
Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60:769–779
Heien M, Khan A, Ariansen J, Cheer J, Phillips P, Wassum K, Wightman M (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A 102:10023–10028
Rao PS, Rujikarn N, Luber JM, Tyras DH (1989) A specific sensitive HPLC method for determination of plasma dopamine. Chromatographia 28:307–310
Peaston RT, Weinkove C (2004) Measurement of catecholamines and their metabolites. Ann Clin Biochem 41:17–38
Nezhad MRH, Tashkhourian J, Khodaveisi J (2010) Sensitive spectrophotometric detection of dopamine. Levodopa and adrenaline using surface plasmon resonance band of silver nanoparticles. J Iran Chem Soc 7:83–91
Lai GS, Zhang HL, Han DY (2008) Electrocatalytic oxidation and voltammetric determination of dopamine at a Nafion/carbon-coated iron nanoparticles-chitosan composite film modified electrode. Microchim Acta 160:233–239
Chang HY, Kim DI, Park YC (2006) Electrochemically degraded dopamine film for the determination of dopamine. Electroanalysis 18:1578–1583
Li SJ, Deng DH, Shi Q, Liu SR (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177:325–331
Chang JL, Wei GT, Zen JM (2011) Screen-printed ionic liquid/ preanodized carbon electrode: effective detection of dopamine in the presence of high concentration of ascorbic acid. Electrochem Commun 13:174
Snowden ME, Unwin PR, Macpherson JV (2011) Single walled carbon nanotube channel flow electrode: hydrodynamic voltammetry at the nanomolar level. Electrochem Commun 13:186
Wang Y, Li YM, Tang LH, Lu J, Li JH (2009) Application of graphene-modified electrode for selective detection of dopamine. Electrochem Commun 11:889
Thiagarajan S, Yang RF, Chen SM (2009) Palladium nanoparticles modified electrode for the selective detection of catecholamine neurotransmitters in presence of ascorbic acid. Bioelectrochemistry 75:163–169
Ciszewski A, Milczarek G (1999) Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic acid. Anal Chem 71:1055
** GY, Zhang YZ, Cheng WX (2005) Poly(p-aminobenzene sulfonic acid)-modified glassy carbon electrode for simultaneous detection of dopamine and ascorbic acid. Sensors Actuators B 107:528
Zhang WE, Xu B, Jiang LC (2010) Functional hybrid materials based on carbon nanotubes and metal oxides. J Mater Chem 20:6383–6391
Hou S, Kasner ML, Su S, Patel K, Cuellari R (2010) Highly sensitive and selective dopamine biosensor fabricated with silanized graphene. J Phys Chem C 114:14915–14921
Liu C, Wang K, Luo S, Tang Y, Chen L (2011) Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 7:1203–1206
Liu R, Zhou H, Liu J, Yao Y, Huang Z, Fu C, Kuang Y (2013) Preparation of Pd/MnO2-reduced graphene oxide nanocomposite for methanol electro-oxidation in alkaline media. Electrochem Commun 26:63–66
Chen ZH, Jie JS, Luo LB, Wang H, Lee CS, Lee ST (2007) Applications of silicon nanowires functionalized with palladium nanoparticles in hydrogen sensors. Nanotechnology 18:345502–345507
Palanisamy S, Chen SM, Sarawathi R (2012) A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sensors Actuators B 166–167:372–377
Lu LM, Li HB, Qu F, Zhang XB, Shen GL, Yu RQ (2011) In situ synthesis of palladium nanoparticle–graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens Bioelectron 26:3500–3504
Nagaraju DH, Suresh GS (2012) Green chemistry route to the synthesis of palladium nanoparticles on reduced graphene oxide for ethanol fuel cells applications. ECS Electrochem Lett 1:21–23
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Jiang Y, Lu Y, Li F, Wu T, Niu L, Chen W (2012) Facile electrochemical codeposition of “clean” graphene–Pd nanocomposite as an anode catalyst for formic acid electrooxidation. Electrochem Commun 19:21–24
Lai GS, Zhang HL, ** GM (2007) Electrocatalysis and voltammetric determination of dopamine at a calix[4]arene crown-4 ether modified glassy carbon electrode. Electroanalysis 19:496–501
Aoki K, Tokuda K, Matsuda H (1987) Theory of stationary current-potential curves at micro disk electrodes for quasi-reversible and totally irreversible electrode reactions. J Electroanal Chem 235:87–96
Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, Kim JS, Kim H (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25:2366–2369
Wang Y, Peng W, Liu L, Tang M, Gao F, Li M (2011) Enhanced conductivity of a glassy carbon electrode modified with a graphene-doped film of layered double hydroxides for selectively sensing of dopamine. Microchim Acta 174:41–46
Zhang F, Li Y, Gu Y, Wang Z, Wang C (2011) One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173:103–109
Wang AJ, Feng J, Li YF, ** JL, Dong WJ (2010) In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis toward dopamine. Microchim Acta 171:431–436
Acknowledgments
This project was supported by the National Science Council and the Ministry of Education of Taiwan (Republic of China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palanisamy, S., Ku, S. & Chen, SM. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim Acta 180, 1037–1042 (2013). https://doi.org/10.1007/s00604-013-1028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1028-1