Abstract
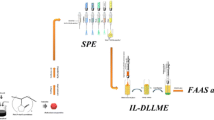
We report on a method for selective extraction and backextraction for the ultra-sensitive determination of Pd(II). Magnetite nanoparticles modified with sodium dodecyl sulfate were used to extract Pd(II) as its green 1-(2-pyridylazo)-2-naphtholate complex prior to zero- and first-derivative spectrophotometric determination at 659 and 681 nm, respectively. A sample volume of 70 mL was backextracted with 0.50 mL of n-butanol in a 3-phasic system. The effects of reaction time and the other variables were optimized. The enrichment factor is 134 and the calibration plots are linear in the range from 2 to 90 ng mL-1 of Pd(II). The detection limit is 0.3 ng mL-1 and the relative standard deviations and recoveries at levels of 10 and 72 ng mL-1 of Pd(II) are in the range from 1.1–4.9%, and from 98.5–102.6%, respectively. Most ions do not significantly interfere. The method was successfully applied to the determination of Pd(II) in water and urine samples, alloys, and palladium catalysts.

The new SPE method was developed for the preconcentration-spectrophotometric determination of palladium using dodecyl sulfate coated magnetite nanoparticles as adsorbrnt and then backextraction by a low volume of n-butanol in a novel 3-phasic backextraction. The established SPE method proved to be efficient for palladium determination and provided satisfactory recoveries and precisions.



Similar content being viewed by others
References
Ozturk N, Bulut VN, Duran C, Soylak M (2011) Coprecipitation of palladium(II) with 1,5-diphenylcarbazite–copper(II) and determination by flame atomic absorption spectrometry. Desalination 270:130–134
Tilch J, Schuster M, Schwarzer M (2000) Determination of palladium in airborne particulate matter in a German city. Fresen J Anal Chem 367:450–453
Boch K, Schuster M, Risse G, Schwarzer M (2002) Microwave-assisted digestion procedure for the determination of palladium in road dust. Anal Chim Acta 459:257–265
Kovacheva P, D**gova R (2002) Ion-exchange method for separation and concentration of platinum and palladium for analysis of environmental samples by inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 464:7–13
Patel KS, Sharma PC, Hoffman P (2000) Graphite furnace-atomic absorption spectrophotometric determination of palladium in soil. Fresen J Anal Chem 367:738–741
Ravindra K, Bencs L, Van Grieken R (2004) Platinum group elements in the environment and their health risk. Sci Tot Environ 318:1–43
Ely JC, Neal CR, Kulpa CF, Schneegurt MA, Seidler JA, Jain JC (2001) Implications of platinum-group element accumulation along U.S. roads from catalytic converter attrition. Environ Sci Technol 35:3816–3822
Van de Velde K, Barbante C, Cozzi G, Moret I, Bellomi T, Ferrari C, Boutron C (2000) Changes in the occurrence of silver, gold, platinum, palladium and rhodium in Mont Blanc ice and snow since the 18th century. Atmos Environ 34:3117–3127
Borges DLG, Silva da Veiga MAM, Frescura VLA, Welz B, Curtius AJ (2003) Cloud-point extraction for the determination of Cd, Pb and Pd in blood by electrothermal atomic absorption spectrometry, using Ir or Ru as permanent modifiers. J Anal At Spectrom 18:501–507
Zeini Jahromi E, Bidari A, Assadi Y, Milani Hosseini MR, Jamali MR (2007) Anal Chim Acta 585:305–311
Malyutina TM, Alekseeva TY, Dyachkova AV, Kudryavtseva GS, Berliner LD, Karpov YA (2010) Dispersive liquid–liquid microextraction combined with graphite furnace atomic absorption spectrometry: ultra trace determination of cadmium in water samples. Inorg Mater 46:1479–1482
Imamoglu M, Aydin AO, Dundar MS (2005) Determination of gold, palladium and copper by flame atomic absorption spectrometry after preconcentration on silica gel modified with 3-(2-aminoethylamino) propyl group. J Chem 3:252–262
Krishna MV, Ranjit M, Chandrasekaran K, Venkateswarlu G, Karunasagar D (2009) On-line preconcentration and recovery of palladium from waters using polyaniline (PANI) loaded in mini-column and determination by ICP-MS; elimination of spectral interferences. Talanta 79:1454–1463
Van Meel K, Smekens A, Behets M, Kazandjian P, Van Grieken R (2007) Determination of platinum, palladium, and rhodium in automotive catalysts using high-energy secondary target X-ray fluorescence spectrometry. Anal Chem 79:6383–6389
Jamali MR, Assadi Y, Rahnama Kozani R, Shemirani F (2009) Homogeneous liquid-liquid extraction method for selective separation and preconcentration of trace amounts of palladium. E-J Chem 6:1077–1084
Ghaedi M, Shokrollahi A, Niknam K, Niknam E, Nijibi A, Soylak M (2009) Cloud point extraction and flame atomic absorption spectrometric determination of cadmium(II), lead(II), palladium(II) and silver(I) in environmental samples. J Hazard Mater 168:1022–1027
Ahmadzadeh Kokya T, Farhadi K (2009) Optimization of dispersive liquid–liquid microextraction for the selective determination of trace amounts of palladium by flame atomic absorption spectroscopy. J Hazard Mater 169:726–733
Rastegarzadeh S, Purreza N, Kiasat AR, Yahyavi H (2010) Selective solid phase extraction of palladium by adsorption of its 5(p-dimethylaminobenzylidene)rhodanine complex on silica-PEG as a new adsorbent. Microchim Acta 170:135–140
Chakrapani G, Mahanta PL, Murty DSR, Gomathy B (2001) Preconcentration of traces of gold, silver and palladium on activated carbon and its determination in geological samples by flame AAS after wet ashing. Talanta 53:1139–1147
Kang SW, Lee SS (1983) The extraction of palladium by polyurethane foam impregnated with β-diphenylglyoxime. J Korean Chem Soc 27:268–72
Elci L, Soylak M, Buyuksekerci EB (2003) Separation of gold, palladium and platinum from metallurgical samples using an amberlite XAD-7 resin column prior to their atomic absorption spectrometric determinations. Anal Sci 19:1621–1624
Tokalioglu S, Oymak T, Kartal S (2004) Determination of palladium in various samples by atomic absorption spectrometry after preconcentration with dimethylglyoxime on silica gel. Anal Chim Acta 511:255–260
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–177
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyl sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831–836
White BR, Stackhouse BT, Holcombe JA (2009) Magnetic γ-Fe2O3 nanoparticles coated with poly-l-cysteine for chelation of As(III), Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). J Hazard Mater 161:848–853
Karatapanis AE, Fiamegos Y, Stalikas CD (2011) Silica-modified magnetic nanoparticles functionalized with cetylpyridinium bromide for the preconcentration of metals after complexation with 8-hydroxyquinoline. Talanta 84:834–839
Suleiman J, Hu B, Peng H, Hung C (2009) Separation/preconcentration of trace amounts of Cr, Cu and Pb in environmental samples by magnetic solid-phase extraction with bismuthiol-II-immobilized magnetic nanoparticles and their determination by ICP-OES. Talanta 77:1579–1583
Li Q, Lama MHW, Wu RSS, Jiang B (2010) Rapid magnetic-mediated solid-phase extraction and pre-concentration of selected endocrine disrupting chemicals in natural waters by poly(divinylbenzene-co-methacrylic acid) coated Fe3O4 core-shell magnetite microspheres for their liquid chromatography-tandem mass spectrometry determination. J Chromatogr A 1217:1219–1226
Zhang S, Niu H, Hu Z, Cai Y, Shi Y (2010) Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A 1271:4757–4764
Gao J, Peng B, Fan H, Kang J, Wang X (1997) Spectrophotometric determination of palladium after solid-liquid extraction with 1-(2-pyridylazo)-2-naphthol at 90 °C. Talanta 44:837–842
Dong Y, Gai K (2005) Spectrophotometric determination of palladium after solid-liquid extraction with 4-(2-pyridylazo)-resorcinol at 90 °C. Bull Korean Chem Soc 25:943–946
Eskandari H (2006) H-point standard addition method–first derivative spectrophotometry for simultaneous determination of palladium and cobalt. Spectrochim Acta A 63:391–397
Bagherian G, Arab Chamjangali M, Eskandari H (2005) Simultaneous determination of cobalt and palladium in micellar media using H-point standard addition method and partial least square regression. Spectrochim Acta A 67:378–384
Bassett J, Denney RC, Jeffery GH, Mendham J (1989) Textbook of quantitative inorganic analysis. Longman Group Limited, New York
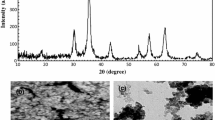
Rossi LM, Vono LLR, Silva FP, Kiyohara PK, Duarte EL, Matos JR (2007) A magnetically recoverable scavenger for palladium based on thiol-modified magnetite nanoparticles. Appl Catal A: Gen 330:139–144
Qazi SJS, Rennie AR, Cockcroft JK, Vickers M (2009) Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J Colloid Interface Science 338:105–110
Myers D (2006) Surfactant science and technology. Wiley, New Jersey
Lide DR (2002) CRC handbook of chemistry and physics. Chapman and Hall/CRC, London
Anthemidis AN, Themelis DG, Stratis JA (2000) Selective stopped-flow injection spectrophotometric determination of palladium(II) in hydrogenation and automobile exhaust gas converter catalysts. Anal Chim Acta 412:161–167
Shemirani F, Rahnama Kozani R, Jamali MR, Assadi Y, Milani Hosseini MR (2006) Cloud-point extraction, preconcentration, and spectrophotometric determination of palladium in water samples. Int J Environ Anal Chem 86:1105–1112
Mohamadi M, Mostafavi A (2010) A novel solidified floating organic drop microextraction based on ultrasound-dispersion for separation and preconcentration of palladium in aqueous samples. Talanta 81:309–313
Yuan CG, Zhang Y, Wang S, Chang A (2011) Separation and preconcentration of palladium using modified multi-walled carbon nanotubes without chelating agent. Microchim Acta 173:361–367
Tokalioglu S, Yilmaz V, Kartal S, Delibas A, Soykan C (2009) Solid phase extraction of Pd(II) on a newly synthesized chelating resin prior to determination by flame atomic absorption spectrometry. Microchim Acta 165:347–352
Acknowledgement
The authors wish to thank the Research Council of the University of Mohaghegh Ardabili for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
Sensitive and selective spectrophotometric determination of palladium(II) ion following its preconcentration using modified magnetite nanoparticles and 3-phasic backextraction (DOC 164 kb)
Rights and permissions
About this article
Cite this article
Eskandari, H., Khoshandam, M. Sensitive and selective spectrophotometric determination of palladium(II) ion following its preconcentration using modified magnetite nanoparticles and 3-phasic backextraction. Microchim Acta 175, 291–299 (2011). https://doi.org/10.1007/s00604-011-0674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0674-4