Abstract
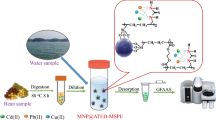
We describe a novel adsorbent for effective extraction of lead(II), chromium(III) and copper(II). It consists of ruthenium nanoparticles loaded on activated carbon that were modified with N,N-bis-(α-methylsalicylidene)-2,2-dimethylpropane-1,3-diamine. The sorbent was applied to solid-phase extraction combined with ionic-liquid based dispersive liquid-liquid microextraction method. The effects of parameters such as amounts of adsorbent, type and volume of elution solvent, type and volume of extraction and dispersing solvents, etc. were evaluated. The ions were then quantified by flame atomic absorption spectrometry. Under the best conditions, limits of detection, linear dynamic ranges and enrichment factors for these ions ranged from 0.02 to 0.09 μg L−1, 0.08 to 45 μg L−1 and 328 to 356, respectively. The results showed that the method, in addition to its sensitivity, selectivity and good enrichment factor, is simple and efficient. It was applied to the determination of the three ions in blood plasma, food (broccoli, coriander and spinach), and in (spiked) samples of tap, spring and river water.

Solid-phase extraction coupled with ionic liquid-based liquid-liquid microextraction using modified ruthenium nanoparticles loaded on activated carbon.


Similar content being viewed by others
References
Banci L, Bertini I, Cantini F, Ciofi-Baffoni S (2010) Cellular copper distribution: a mechanistic systems biology approach. Cell Mol Life Sci 67:2563–2589
Yousefi SM, Shemirani F (2013) Selective and sensitive speciation analysis of Cr (VI) and Cr (III) in water samples by fiber optic-linear array detection spectrophotometry after ion pair based-surfactant assisted dispersive liquid–liquid microextraction. J Hazard Mater 254:134–140
Oymak T, Tokalıoğlu Ş, Yılmaz V, Kartal Ş, Aydın D (2009) Determination of lead and cadmium in food samples by the coprecipitation method. Food Chem 113:1314–1317
Rajabi M, Asemipour S, Barfi B, Jamali MR, Behzad M (2014) Ultrasound-assisted ionic liquid based dispersive liquid–liquid microextraction and flame atomic absorption spectrometry of cobalt, copper, and zinc in environmental water samples. J Mol Liq 194:166–171
Yilmaz V, Arslan Z, Hazer O, Yilmaz H (2014) Selective solid phase extraction of copper using a new Cu (II)-imprinted polymer and determination by inductively coupled plasma optical emission spectroscopy (ICP-OES). Microchem J 114:65–72
Pinto JJ, Moreno C, García-Vargas M (2002) A simple and very sensitive spectrophotometric method for the direct determination of copper ions. Anal Bioanal Chem 373:844–848
Labrecque C, Potvin S, Whitty-Léveillé L, Larivière D (2013) Cloud point extraction of uranium using H2DEH[MDP] in acidic conditions. Talanta 107:284–291
Aouarram A, Galindo-Riaño M, García-Vargas M, Stitou M, El Yousfi F (2007) A permeation liquid membrane system for determination of nickel in seawater. Talanta 71:165–170
Efendioğlu A, Yağan M, Batı B (2007) Bi (III) 4-methylpiperidinedithiocarbamate coprecipitation procedure for separation–pre-concentration of trace metal ions in water samples by flame atomic absorption spectrometric determination. J Hazard Mater 149:160–165
Rajabi M, Barfi B, Asghari A, Najafi F, Aran R (2014) Hybrid amine-functionalized titania/silica nanoparticles for solid-phase extraction of lead, copper, and zinc from food and water samples: kinetics and Equilibrium studies. Food Anal Methods. doi:10.1007/s12161-014-9964-x
Zhou Q, **ng A, Zhao K (2014) Simultaneous determination of nickel, cobalt and mercury ions in water samples by solid phase extraction using multiwalled carbon nanotubes as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. J Chromatogr A 1360:76–81
Camel V (2003) Solid phase extraction of trace elements. Spectrochim Acta B 58:1177–1233
He Q, Hu Z, Jiang Y, Chang X, Tu Z, Zhang L (2010) Preconcentration of Cu (II), Fe (III) and Pb (II) with 2-((2-aminoethylamino) methyl) phenol-functionalized activated carbon followed by ICP-OES determination. J Hazard Mater 175:710–714
Kikuchi Y, Qian Q, Machida M, Tatsumoto H (2006) Effect of ZnO loading to activated carbon on Pb (II) adsorption from aqueous solution. Carbon 44:195–202
Karimipour G, Ghaedi M, Sahraei R, Daneshfar A, Biyareh MN (2012) Modification of gold nanoparticle loaded on activated carbon with bis (4-methoxysalicylaldehyde)-1, 2-phenylenediamine as new sorbent for enrichment of some metal ions. Biol Trace Elem Res 145:109–117
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Hu B, He M, Chen B, **a L (2013) Liquid phase microextraction for the analysis of trace elements and their speciation. Spectrochim Acta B 86:14–30
Anderson JL, Armstrong DW, Wei GT (2006) Ionic liquids in analytical chemistry. Anal Chem 78:2892–2902
Rajabi M, Haji-Esfandiari S, Barfi B, Ghanbari H (2014) Ultrasound-assisted temperature-controlled ionic-liquid dispersive liquid-phase microextraction method for simultaneous determination of anethole, estragole, and para-anisaldehyde in different plant extracts and human urine: a comparative study. Anal Bioanal Chem 460:4501–4512
Wen S, Wu J, Zhu X (2013) Room temperature ionic liquid-based dispersive liquid–liquid microextraction combined with flame atomic absorption spectrometry for the speciation of chromium (III) and chromium (VI). J Mol Liq 180:59–64
Rao RN, Raju SS, Vali RM (2013) Ionic-liquid based dispersive liquid–liquid microextraction followed by high performance liquid chromatographic determination of anti-hypertensives in rat serum. J Chromatogr B 931:174–180
He L, Luo X, Jiang X, Qu L (2010) A new 1, 3-dibutylimidazolium hexafluorophosphate ionic liquid-based dispersive liquid–liquid microextraction to determine organophosphorus pesticides in water and fruit samples by high-performance liquid chromatography. J Chromatogr A 1217:5013–5020
Shamsipur M, Fattahi N, Sadeghi M, Pirsaheb M (2014) Determination of ultra traces of lead in water samples after combined solid-phase extraction–dispersive liquid–liquid microextraction by graphite furnace atomic absorption spectrometry. J Iran Chem Soc 11:249–256
Shamsipur M, Fattahi N, Assadi Y, Sadeghi M, Sharafi K (2014) Speciation of as (III) and as (V) in water samples by graphite furnace atomic absorption spectrometry after solid phase extraction combined with dispersive liquid-liquid microextraction based on the solidification of floating organic drop. Talanta 130:26–32
Salavati-Niasari M, Salemi P, Davar F (2005) Oxidation of cyclohexene with tert-butylhydroperoxide and hydrogen peroxide catalyzed by Cu (II), Ni (II), Co (II) and Mn (II) complexes of N, N-bis-(α-methylsalicylidene)-2,2-dimethylpropane-1,3-diamine supported on alumina. J Mol Catal A Chem 238:215–222
Joo SH, Park JY, Renzas JR, Butcher DR, Huang W, Somorjai GA (2010) Size effect of ruthenium nanoparticles in catalytic carbon monoxide oxidation. Nano Lett 10:2709–2713
Sahraei R, Motedayen Aval G, Goudarzi A (2008) Compositional, structural, and optical study of nanocrystalline ZnS thin films prepared by a new chemical bath deposition route. J Alloys Compd 466:488–492
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911–920
Salarian M, Ghanbarpour A, Behbahani M, Bagheri S, Bagheri A (2014) A metal-organic framework sustained by a nanosized Ag12 cuboctahedral node for solid-phase extraction of ultra traces of lead(II) ions. Microchim Acta 181:999–1007
Hajiaghababaei L, Tajmiri T, Badiei A, Ganjali MR, Khaniani Y, Ziarani GM (2013) Heavy metals determination in water and food samples after preconcentration by a new nanoporous adsorbent. Food Chem 141:1916–1922
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Taghizadeh M, Asgharinezhad AA, Samkhaniany N, Tadjarodi A, Abbaszadeh A, Pooladi M (2014) Solid phase extraction of heavy metal ions based on a novel functionalized magnetic multi-walled carbon nanotube composite with the aid of experimental design methodology. Microchim Acta 181:597–605
Acknowledgments
The authors would like to thank Semnan University Research Council for financial support of this work.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 69 kb)
Rights and permissions
About this article
Cite this article
Barfi, B., Rajabi, M., Zadeh, M.M. et al. Extraction of ultra-traces of lead, chromium and copper using ruthenium nanoparticles loaded on activated carbon and modified with N,N-bis-(α-methylsalicylidene)-2,2-dimethylpropane-1,3-diamine. Microchim Acta 182, 1187–1196 (2015). https://doi.org/10.1007/s00604-014-1434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1434-z