Abstract
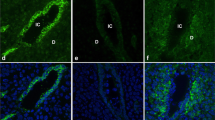
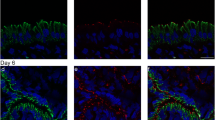
During the estrous cycle, the endometrium epithelium experiences marked cellular structural changes. For fertilization to proceed, maintenance of an adequate uterine environment by ovarian hormones is essential. Epithelial cells lining the uterine lumen are associated with each other by tight junctions (TJs), which regulate the passage of ions and molecules through the paracellular pathway. The aim of the present study was to assess by confocal immunofluorescence the distribution pattern of the TJ proteins ZO-1, occludin, and claudins 1–7 in the rat uterus during the estrous cycle. Our results reveal that on proestrus, the day when mating takes place, ZO-1, occludin, and claudins 1 and 5 are located in the TJs, while claudins 3 and 7 display a basolateral distribution. In contrast, on metestrus day, when no sexual mating occurs and the uterine lumen is devoid of secretions, none of these proteins were detected in the TJ region, and only a diffuse cytosolic staining was observed for some of the proteins. On estrus and diestrus days, an intermediate situation was encountered, since ZO-1 localized in the TJs, whereas occludin was no longer detectable in the TJs. The distribution of claudins during these stages varied from the lowermost portion of the basolateral membrane to its apex. In conclusion, the results show that the protein composition of TJs present in the luminal epithelial cells of the uterus changes during the different days of the estrous cycle, and suggest that the expression of TJ proteins participates in providing an adequate environment for a successful fertilization.









Similar content being viewed by others
References
Alavi N, Lianos EA, Palant CE, Bentzel CJ (1983) Induction of epithelial tight junctions by a light chain protein isolated from a patient with Fanconi’s syndrome. Nephron 35:130–135
Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y (1999) Similar and differential behaviour between the nectin–afadin–ponsin and cadherin–catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4:573–581
Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M (1991) Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol 122:193–202
Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM (1993) Assembly of the tight junction: the role of diacylglycerol. J Cell Biol 123:293–302
Boulpaep EL, Seely JF (1971) Electrophysiology of proximal and distal tubules in the autoperfused dog kidney. Am J Physiol 221:1084–1096
Bourroughs KD, Fuchs-Young R, Davis B, Walker CL (2000) Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Rep 63:1322–1330
Calderon V, Lazaro A, Contreras RG, Shoshani L, Flores-Maldonado C, Gonzalez-Mariscal L, Zampighi G, Cereijido M (1998) Tight junctions and the experimental modifications of lipid content. J Membr Biol 164:59–69
Cameron VA, Autelitano DJ, Evans JJ, Ellmers LJ, Espiner EA, Nicholls MG, Richards AM (2002) Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol Endocrinol Metab 282:E139–E146
Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG (2000) Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta 1493:319–324
Cohen E, Talmon A, Faff O, Bacher A, Ben Shaul Y (1985) Formation of tight junctions in epithelial cells, I: induction by proteases in a human colon carcinoma cell line. Exp Cell Res 156:103–116
Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM (2002) Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283:C142–C147
Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284:C1346–C1354
Contreras RG, Miller JH, Zamora M, Gonzalez-Mariscal L, Cereijido M (1992) Interaction of calcium with plasma membrane of epithelial (MDCK) cells during junction formation. Am J Physiol 263:C313–C318
Frömter E, Diamond J (1972) Route of passive ion permiation in epithelia. Nat N Biol 235:9–13
Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111
Ghassemifar MR, Sheth B, Papenbrock T, Leese HJ, Houghton FD, Fleming TP (2002) Occludin TM4(−): an isoform of the tight junction protein present in primates lacking the fourth transmembrane domain. J Cell Sci 115:3171–3180
Gilula NB, Fawcett DW, Aoki A (1976) The Sertoli cell occluding junctions and gap junctions in mature and develo** mammalian testis. Dev Biol 50:142–168
Gonzalez-Mariscal L, Chavez dR, Cereijido M (1984) Effect of temperature on the occluding junctions of monolayers of epithelioid cells (MDCK). J Membr Biol 79:175–184
Gonzalez-Mariscal L, Chavez dR, Cereijido M (1985) Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol 86:113–125
Gonzalez-Mariscal L, Contreras RG, Bolivar JJ, Ponce A, Chavez dR, Cereijido M (1990) Role of calcium in tight junction formation between epithelial cells. Am J Physiol 259:C978–C986
Gonzalez-Mariscal L, Betanzos A, Avila-Flores A (2000a) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324
Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL (2000b) Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 57:2386–2402
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE (2003) Tight junction proteins. Prog Biophys Mol Biol 81:1–44
Gorodeski GI (2001) Estrogen biphasic regulation of paracellular permeability of cultured human vaginal-cervical epithelia. J Clin Endocrinol Metab 86:4233–4243
Hegel U, Frömter E, Wick T (1967) Der elektrische Wandwiderstand des proximalen Konvolutes der Ratteniere. Pflügers Arch 294:274–290
Helman SI, Grantham JJ, Burg MB (1971) Effect of vasopressin on electrical resistance of renal cortical collectin tubules. Am J Physiol 220:1825–1832
Hoover KB, Liao SY, Bryant PJ (1998) Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol 153:1767–1773
Isobe T, Minoura H, Tanaka K, Shibahara T, Hayashi N, Toyoda N (2002) The effect of RANTES on human sperm chemotaxis. Hum Reprod 17:1441–1446
Iwanaga S, Inokuchi T, Notohara A, Higashi R, Murakami M, Kato T (1985) Alterations in tight junctions of human endometrial epithelial cells during normal menstrual cycle—freeze-fracture electron microscopic study. Nippon Sanka Fu**ka Gakkai Zasshi 37:2847–2852
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886
Kobayashi T, Kaneko T, Iuchi Y, Matsuki S, Takahashi M, Sasagawa I, Nakada T, Fujii J (2002) Localization and physiological implication of aldose reductase and sorbitol dehydrogenase in reproductive tracts and spermatozoa of male rats. J Androl 23:674–683
Kramer F, White K, Kubbies M, Swisshelm K, Weber BH (2000) Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Hum Genet 107:249–256
Lapointe S, Bilodeau JF, Lemieux D, Asselin E, Fortier MA, Sirard MA (2000) Epithelial and stromal uterine cells cultured in vitro protect bovine sperm from hydrogen peroxide. Theriogenology 54:355–369
Lechner F, Sahrbacher U, Suter T, Frei K, Brockhaus M, Koedel U, Fontana A (2000) Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J Infect Dis 182:978–982
Li D, Mrsny RJ (2000) Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 148:791–800
Mendoza-Rodriguez CA, Merchant-Larios H, Segura-Valdez Md ML, Moreno-Mendoza N, Cruz ME, Arteaga-Lopez P, Camacho-Arroyo I, Dominguez R, Cerbon M (2002) Expression of p53 in luminal and glandular epithelium during the growth and regression of rat uterus during the estrous cycle. Mol Reprod Dev 61:445–452
Mendoza-Rodriguez CA, Merchant-Larios H, Segura-Valdez ML, Moreno-Mendoza N, Cruz ME, Arteaga-Lopez P, Camacho-Arroyo I, Dominguez R, Cerbon M (2003a) c-fos and estrogen receptor gene expression pattern in the rat uterine epithelium during the estrous cycle. Mol Reprod Dev 64:379–388
Mendoza-Rodriguez CA, Monroy-Mendoza MG, Morimoto S, Cerbon MA (2003b) Pro-apoptotic signals of the bcl-2 gene family in the rat uterus occurs in the night before the day of estrus and precedes ovulation. Mol Cell Endocrinol 208:31–39
Molnar P, Murphy LJ (1994) Effects of oestrogen on rat uterine expression of insulin-like growth factor-binding proteins. J Mol Endocrinol 13:59–67
Muresan Z, Paul DL, Goodenough DA (2000) Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell 11:627–634
Murphy CR, Swift JG, Mukherjee TM, Rogers AW (1981) Effects of ovarian hormones on cell membranes in the rat uterus. II. Freeze-fracture studies on tight junctions of the lateral plasma membrane of the luminal epithelium. Cell Biophys 3:57–69
Murphy CR, Swift JG, Mukherjee TM, Rogers AW (1982) The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell Tissue Res 223:281–286
Nandha KA, Benito-Orfila MA, Jamal H, Akinsanya KO, Bloom SR, Smith DM (1999) Effect of steroids and the estrous cycle on uterine neuromedin U receptor expression. Peptides 20:1203–1209
Nguyen DD, Beeman N, Neville MC (2003) Regulation of tight junction permeability in the mammary gland. In: Cereijido M, Anderson JM (eds) Tight junctions. CRC, Boca Raton, pp 395–414
Nilsson O (1958a) Influence of estradiol on the ultrastructure of mouse uterine surface epithelium. Exp Cell Res 14:434–435
Nilsson O (1958b) Ultrastructure of mouse uterine surface epithelium under different estrogenic influences, 1: spayed animals and oestrous animals. J Ultrastruct Res 1:375–396
Ojeda ER, Urbanski HF (1994) Puberty in the rat. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven, New York, pp 363–410
Orchard MD, Murphy CR (2002) Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem 104:149–155
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Rau WS, Frömter E (1974) Electrical properties of the medullary ducts of the golden hamster kidney. Pflügers Arch 351:113–131
Reyes JL, Roch-Ramel F, Besseghir K (1987) Net sodium and water movements in the newborn rat collecting tubule: lack of modifications by indomethacin. Biol Neonate 51:212–216
Reyes JL, Lamas M, Martin D, del Carmen NM, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L (2002) The renal segmental distribution of claudins changes with development. Kidney Int 62:476–487
Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP (1997) Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha + isoform. Development 124:2027–2037
Simon DB, Lu Y, Choate KA, Velazquez H, Al Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Van Itallie C, Rahner C, Anderson JM (2001) Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107:1319–1327
Van Itallie CM, Fanning AS, Anderson JM (2003) Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285:F1078–F1084
Wang XF, Yu MK, Lam SY, Leung KM, Jiang JL, Leung PS, Ko WH, Leung PY, Chew SB, Liu CQ, Tse CM, Chan HC (2003) Expression, immunolocalization, and functional activity of Na+/H+ exchanger isoforms in mouse endometrial epithelium. Biol Reprod 68:302–308
Williams T, Rogers AW (1972) Morphological changes in the luminal epithelium of the rat uterus in response to progesterone and oestradiol. J Anat 11:515
Yonemura S, Itoh M, Nagafuchi A, Tsukita S (1995) Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108(Pt 1):127–142
Yu AS, Enck AH, Lencer WI, Schneeberger EE (2003) Claudin-8 expression in Madin–Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278:17350–17359
Acknowledgements
The authors wish to thank Socorro Islas, Jose Luna, and Montserrat García for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants PAPIIT (IN210902, IX228504) and PAIP (6190-08) from the National Autonomous University of Mexico (UNAM), and by grants G34511-M and 37846-N from the Mexican National Council on Science and Technology (CONACYT).
Rights and permissions
About this article
Cite this article
Mendoza-Rodríguez, C.A., González-Mariscal, L. & Cerbón, M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res 319, 315–330 (2005). https://doi.org/10.1007/s00441-004-1010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1010-7