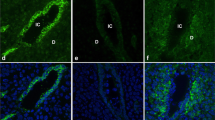
Abstract
Uterine luminal epithelial cells (UECs) undergo the plasma membrane transformation in the transition to receptivity. This involves transient alterations in the apical junctional complex (AJC) including increases to the depth and complexity of the tight junction, loss of the adherens junction, and a decrease in the number of desmosomes along the lateral cell membranes. Nectin-3 is key protein involved in the structure and function of the AJC. This study, used immunofluorescence, Western blotting, colocalization, and coimmunoprecipitation analyses, to investigate whether nectin-3 was present in the rat uterus and was regulated by hormones and the blastocyst during early pregnancy. The results showed that nectin-3 was present in UECs as 3 molecular weight protein isoforms (80 kDa, 60 kDa, and 32 kDa). At the time of fertilization (day 1 of pregnancy), nectin-3 was localized basally, but at the time of implantation, (day 6 of pregnancy) when UECs were receptive, nectin-3 increased in the cellular junctions. When UECs returned to the nonreceptive state (day 9 of pregnancy), nectin-3 redistributed back to the cell cytoplasm. This study also showed that nectin-3 localization at the cell junctions was likely to be controlled by progesterone; however, neither ovarian hormones nor the blastocyst regulated protein abundance. This study further showed that while nectin-3 localized to the tight junction at the time of implantation, it did not interact with occludin or l-afadin. These results suggest that at the time of implantation, nectin-3 may contribute to the formation of the tight junction in a protein complex independent from occludin and l-afadin.
Similar content being viewed by others
References
Murphy CR. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004;14(4):259–267.
Finn CA, Martin L. The control of implantation. Reproduction. 1974;39(1):195–206.
Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72(12):1448–1458. doi:10.1038/sj.ki.5002579.
Farquhar MG. Junctional complexes in various epithelia. J Cell Biol. 1963;17(2):375–412. doi:10.1083/jcb.17.2.375.
Furuse M. Molecular Basis of the Core Structure of Tight Junctions. Cold Spring Harbor Perspectives in Biology. 2010;2(1):a002907. doi:10.1101/cshperspect.a002907.
Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi:10.1038/35067088.
Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi:10.1016/j.bbamem.2007.07.012.
Murphy CR, Swift JG, Mukherjee TM, Rogers AW. The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell Tissue Res. 1982;223(2):281–286.
Murphy CR. Junctional barrier complexes undergo major alterations during the plasma membrane transformation of uterine epithelial cells. Hum Reprod. 2000;15(suppl 3):182–188.
Preston AM, Lindsay LA, Murphy CR. Desmosomes in uterine epithelial cells decrease at the time of implantation: an ultrastructural and morphometric study. J Morphol. 2006;267(1):103–108.
Albaghdadi AJH, Kan FWK. Endometrial receptivity defects and impaired implantation in diabetic NOD mice. Biol Reprod. 2012;87(2):30.
Ghosh D, Danielson KG, Alston JT, Heyner S. Functional differentiation of mouse uterine epithelial cells grown on collagen gels or reconstituted basement membranes. In Vitro Cell Dev Biol. 1991;27A(9):713–719.
Illingworth IM, Kiszka I, Bagley S, Ireland GW, Garrod DR, Kimber SJ. Desmosomes are reduced in the mouse uterine luminal epithelium during the preimplantation period of pregnancy: a mechanism for facilitation of implantation. Biol Reprod. 2000;63(6):1764–1773.
Johnson SA, Morgan G, Wooding FB. Alterations in uterine epithelial tight junction structure during the oestrous cycle and implantation in the pig. J Reprod Fertil. 1988;83(2):915–922.
Bowen JA, Newton GR, Weise DW, Bazer FW, Burghardt RC. Characterization of a polarized porcine uterine epithelial model system. Biol Reprod. 1996;55(3):613–619.
Mani SK, Decker GL, Glasser SR. Hormonal responsiveness by immature rabbit uterine epithelial cells polarized in vitro. Endocrinology. 1991;128(3):1563–1573.
Winterhager E, Kühnel W. Alterations in intercellular junctions of the uterine epithelium during the preimplantation phase in the rabbit. Cell Tissue Res. 1982;224(3):517–526.
Winterhager E, Mendoza AS. Structure of quick-frozen tight junctions in uterine epithelium of pseudopregnant rabbits. Z Mikrosk Anat Forsch. 1987;101(1):179–185.
Murphy CR, Swift JG, Need JA, Mukherjee TM, Rogers AW. A freeze-fracture electron microscopic study of tight junctions of epithelial cells in the human uterus. Anat Embryol (Berl). 1982;163(4):367–370. doi:10.1007/BF00305552.
Murphy CR, Rogers PA, Hosie MJ, Leeton J, Beaton L. Tight junctions of human uterine epithelial cells change during the menstrual cycle: a morphometric study. Acta Anat (Basel). 1992;144(1):36–38.
Someya M, Kojima T, Ogawa M, et al. Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res. 2013;354(2):481–494. doi:10.1007/s00441-013-1676-9.
Nicholson MDO, Lindsay LA, Murphy CR. Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem. 2010;112(1):42–52.
Orchard MD, Murphy CR. Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem. 2002;104(2):149–155.
Hyland RA, Shaw TJ, Png FY, Murphy CR. Pan-cadherin concentrates apically in uterine epithelial cells during uterine closure in the rat. Acta Histochem. 1998;100(1):75–81.
Slater M, Murphy CR, Barden JA. Tenascin, E-cadherin and P2X calcium channel receptor expression is increased during rat blastocyst implantation. Histochem J. 2002;34(1-2):13–19.
Preston AM, Lindsay LA, Murphy CR. Progesterone treatment and the progress of early pregnancy reduce desmoglein 1&2 staining along the lateral plasma membrane in rat uterine epithelial cells. Acta Histochem. 2003;106(5):345–351.
Satoh-Horikawa KK, Nakanishi HH, Takahashi KK, et al. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275(14):10291–10299.
Takai Y. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116(1):17–27.
Kuramitsu K, Ikeda W, Inoue N, Tamaru Y, Takai Y. Novel role of nectin: implication in the co-localization of JAM-A and claudin-1 at the same cell-cell adhesion membrane domain. Genes Cells. 2008;13(8):797–805.
Takai YY, Ikeda WW, Ogita HH, Rikitake YY. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Cell Dev Biol. 2007;24:309–342.
Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11(9):1125–1132. doi:10.1111/j.1365-2443.2006.01006.x.
Ballester M, Gonin J, Rodenas A, et al. Eutopic endometrium and peritoneal, ovarian and colorectal endometriotic tissues express a different profile of Nectin-1, -3, -4 and nectin-like molecule 2. Hum Reprod. 2012;27(11):3179–3186.
Okabe N, Ozaki-Kuroda K, Nakanishi H, Shimizu K, Takai Y. Expression patterns of nectins and afadin during epithelial remodeling in the mouse embryo. Dev Dyn. 2004;230(1):174–186.
Swingle WW, Seay P, Perlmutt J, Collins EJ, George Barlow JR, Fedor EJ. An experimental study of pseudopregnancy in rat. Am J Physiol—Leg Content. 1951;167(3):586–592.
Ljungkvist I. Attachment reaction of rat uterine luminal epithelium. II. The effect of progesterone on the morphology of the uterine glands and the luminal epithelium of the spayed, virgin rat. Acta Soc Med Ups. 1971;76(3-4):110–126.
Ljungkvist I. Attachment reaction of rat uterine luminal epithelium. 3. The effect of estradiol, estrone and estriol on the morphology of the luminal epithelium of the spayed, virgin rat. Acta Soc Med Ups. 1971;76(3-4):139–157.
Murphy CR, Rogers AW. Effects of ovarian hormones on cell membranes in the rat uterus. III. The surface carbohydrates at the apex of the luminal epithelium. Cell Biophys. 1981;3(4):305–320.
Kaneko YY, Lindsay LALA, Murphy CRCR. Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reprod Fertil Dev. 2008;20(8):892–899.
Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256.
Reymond N, Borg JP, Lecocq E, et al. Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene. 2000;255(2):347–355.
Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: Roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94(8):655–667.
Tanaka-Okamoto M, Hori K, Ishizaki H, et al. Involvement of afadin in barrier function and homeostasis of mouse intestinal epithelia. J Cell Sci. 2011;124(pt 13):2231–2240.
Kim J, Chang A, Dudak A, Federoff HJ, Lim ST. Characterization of nectin processing mediated by presenilin-dependent γ-secretase. J Neurochem. 2011;119(5):945–956.
Mizoguchi A, Nakanishi H, Kimura K, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156(3):555–565.
Yamada T, Kuramitsu K, Rikitsu E, Kurita S, Ikeda W, Takai Y. Nectin and junctional adhesion molecule are critical cell adhesion molecules for the apico-basal alignment of adherens and tight junctions in epithelial cells. Genes Cells. 2013;18(11):985–998.
Krause G, Winkler L, Mueller SL, Haseloff RF. Structure and function of claudins. Biochim Biophys Acta. 2008;1778(3):631–645.
Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32(2):242–250.
Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584.
O’Leary S, Jasper MJ, Robertson SA, Armstrong DT. Seminal plasma regulates ovarian progesterone production, leukocyte recruitment and follicular cell responses in the pig. Reproduction. 2006;132(1):147–158.
Martin TA, Lane J, Harrison GM, Jiang WG. The expression of the Nectin complex in human breast cancer and the role of Nectin-3 in the control of tight junctions during metastasis. PLoS One. 2013;8(12):e82696. doi:10.1371/journal.pone.0082696.
Takahashi K. Nectin/PRR: An immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145(3):539–549.
Mandai K, Rikitake Y, Shimono Y, Takai Y. Afadin/AF-6 and canoe: roles in cell adhesion and beyond. Prog Mol Biol Transl Sci. 2013;116:433–454.
Kobayashi R, Kurita S, Miyata M, et al. s-Afadin binds more preferentially to the cell adhesion molecules nectins than l-afadin. Genes to Cells. 2014;19(12):853–863. doi:10.1111/gtc.12185.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poon, C.E., Madawala, R.J., Dowland, S.N. et al. Nectin-3 Is Increased in the Cell Junctions of the Uterine Epithelium at Implantation. Reprod. Sci. 23, 1580–1592 (2016). https://doi.org/10.1177/1933719116648216
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116648216