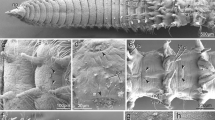
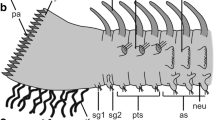
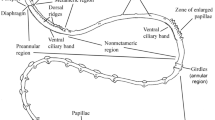
Abstract
Larvae of Poecilochaetus serpens, Trochochaeta multisetosum and Polydora ciliata possess almost identical unpigmented, ciliary, presumptive light sensitive organs within the prostomium. The data corroborate hypotheses on the close relationship of Poecilochaetidae, Trochochaetidae and Spionidae and are even congruent with inclusion of Poecilochaetidae and Trochochaetidae within Spionidae. The organs in P. serpens, T. multisetosum and P. ciliata are composed of one monociliary receptor cell, one supportive cell and several associated flask shaped bipolar sensory cells. The receptor cell cilium enters the supportive cell cavity through a thin pore, dilates and then branches into a high number of disordered projections. The associated sensory cells bear one or occasionally two cilia, which run horizontally beneath or within the cuticle. The supportive cell cavity is not sealed by any cell contact from the subcuticular extracellular space. The organs in Magelona mirabilis are composed of a single supportive cell, but several receptor cells. No further sensory cells are associated. Each receptor cell sends one cilium into an own invagination of the supportive cell, and the ciliary branches are highly ordered. The examined organs in P. serpens, T. multisetosum and P. ciliata exhibit a unique organization amongst polychaetes. The organs of M. mirabilis are most probably homologous. A homology to ciliary organs of Protodrilida is conceivable. In the lineage leading to Protodrilida, primary larval organs may have been integrated into the adult body organization by heterochrony.









Similar content being viewed by others
References
Allen EJ (1904) The anatomy of Poecilochaetus, Claparède. Q J Microsc Sci 48:79–151
Arendt D, Tessmar K, de Campos-Batista M-IM, Dorresteijn A, Wittbrodt J (2002) Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development 129:1143–1154
Arendt D, Teßmar-Raible K, Snyman H, Dorresteijn A, Wittbrodt J (2004) Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 36:869–871
Arendt D, Wittbrodt J (2001) Reconstructing the eyes of Urbilateria. Philos Trans R Soc Lond B 356:1545–1563
Attems C (1902) Beiträge zur Anatomie und Histologie von Scolelepis fuliginosa. Arbeiten aus den Zoologischen Instituten der Universität Wien und der Zoologischen Station in Triest 14:173–210
Bartolomaeus T (1987) Ultrastruktur des Photorezeptors der Trochophora von Anaitides mucosa Oersted (Phyllodocidae, Annelida). Microfauna Mar 3:411–418
Bartolomaeus T (1992) Ultrastructure of the photoreceptors in certain larvae of the Annelida. Microfauna Mar 7:191–214
Bartolomaeus T (1993) Different photoreceptors in juvenile Ophelia rahtkei (Annelida, Opheliida). Microfauna Mar 8:99–114
Blake JA (1996) Family Apistobranchidae Mesnil and Caullery, 1898. In: Blake JA, Hilbig B, Scott PH (eds) The Annelida Part 3—Polychaete: orbiniidae to Cossuridae. Taxonomic Atlas of the benthic fauna of the Santa Maria Basin and the western Santa Barbara Channel, vol 6. Santa Barbara Museum of Natural History, Santa Barbara, pp 71–79
Blake JA, Arnofsky PL (1999) Reproduction and larval development of the spioniform Polychaeta with application to systematics and phylogeny. Hydrobiologia 402:57–106
Dales RP (1963) Annelids. Hutchinson University Library, London
Eakin RM (1963) Lines of evolution of photoreceptors. In: Mazia D, Tyler A (eds) General physiology of cell specialization. MacGraw-Hill, New York, pp 393–425
Eakin RM (1982) Continuity and diversity in photoreceptors. In: Westafall JA (ed) Visual cells in evolution. Raven Press, New York, pp 91–105
Eakin RM, Hermans CO (1988) Eyes. In: Westheide W, Hermans CO (eds) The Ultrastructure of Polychaeta. Microfauna Marina, vol 4. Gustav Fischer Verlag, Stuttgart, Jena, New York
Fauchald K (1977) The polychaete worms. Definitions and keys to the orders, families and genera. Nat Hist Mus Long Angel Cty Sci Ser 28:1–88
Fauchald K, Rouse G (1997) Polychaete systematics: past and present. Zool Scr 26(2):71–138
George JD, Hartmann-Schröder G (1985) Polychaetes: British Amphinomida, Spintherida and Eunicida. Keys and notes for the identification of the species. EJ Brill/Dr. W Backhyus, London
Hannerz L (1956) Larval development of the polychaete families Spionidae Sars, Disomidae Mesnil, and Poecilochaetidae n. fam. in the Gullmar Fjord (Sweden). Zool Bidr Upps 31:1–204
Hausen H (2005) Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia 535/536:37–52
Hausen H, Bartolomaeus T (1998) Setal structure and chaetogenesis in Scolelepis squamata and Malacoceros fuliginosus (Spionidae, Annelida). Acta Zool 79(3):149–161
Hessling R, Purschke G (2000) Immunohistochemical (cLSM) and ultrastructural analysis of the central nervous system and sense organs in Aelosoma hemprichi (Annelida, Aeolosomatidae). Zoomorphology 120:65–78
Holborow PL, Laverack MS (1972) Presumptive photoreceptor structures of the trochophore of Harmothoe imbricata (Polychaeta). Mar Behav Physiol 1:139–156
Marsden JR, Hsieh J (1987) Ultrastructure of the eyespot in three polychaete trochophore larvae (Annelida). Zoomorphology 106:361–368
Mesnil F (1897) Études de morphologie externe chez les annélides. Wiss Meeresuntersuchungen 2:83–100
Müller MCM (1999) Das Nervensystem der Polychaeten: immunhistochemische Untersuchungen an ausgewählten Taxa. Dissertation, Universität Osnabrück, p 402
Niilonen T (1980) Fine Structure of the phaosomous photoreceptor in the larvae of Polydora ligni Webster (Polychaeta: Spionidae). Acta Zool 61:183–190
Orrhage L (1964) Anatomische und morphologische Studien über die Polychaetenfamilien Spionidae, Disomidae und Poecilochaetidae. Zool Bidr Upps 36(3):335–418
Pietsch A, Westheide W (1985) Ultrastructural investigations of presumed photoreceptors as a means of discrimination and identification of closely related species of the genus Microphthalmus (Polychaeta, Hesionidae). Zoomorphology 105:265–276
Purschke G (1990a) Comparative electron microscopic investigation of the nuchal organs in Protodriloides, Protodrilus, and Saccocirrus (Annelida, Polychaeta). Can J Zool 68:325–338
Purschke G (1990b) Fine structure of the so-called statocysts in Protodrilus adhaerens Protodrilidae, Polychaeta). Zool Anz 224(5/6):289–296
Purschke G (1990c) Ultrastructue of the “statocysts” in Protodrilus species (Polychaeta): reconstruction of the cellular organization with morphometric data from receptor cells. Zoomorphology 110:91–104
Purschke G (1992) Ultrastrucural investigations of presumed photoreciptive organs in two Saccocirrus species (Polychaeta, Saccocirridae). J Morphol 211:7–21
Purschke G (1993) Structure of the prostomial appendages and the central nervous system in the Protodrilida (Polychaeta). Zoomorphology 113:1–20
Purschke G (2005) Sense organs in polychaetes (Annelida). Hydrobiologia 535/536:53–78
Purschke G, Hessling R (2002) Analysis of the central nervous system and sense organs in Potamodrilus fluviatilisi (Annelida: Potamodrilidae). Zool Anz 241:19–35
Purschke G, Tzetlin AB (1996) Dorsolateral ciliary folds in the polychaete foregut: structure, prevalence and phylogenetic significance. Acta Zool 77(1):33–49
Rhode B (1991) Ultrastructure of prostomial photoreceptors in four marine polychaete species (Annelida). J Morphol 209:177–188
Rhode B (1993) Larval and adult eyes in Capitella spec. I (Annelida, Polychaeta). J Morphol 217:327–335
Rouse G, Fauchald K (1997) Cladistics and polychaetes. Zool Scr 26(2):139–204
Schlötzer-Schrehardt U (1987) Ultrastructural investigations of the nuchal organs of Pygospio elegans (Polychaeta). II. Adult nuchal and dorsal organs. Zoomorphology 107:169–179
Schlötzer-Schrehardt U (1992) Ultrastrukturelle Untersuchungen zur Reproduktion und Postembryonalentwicklung einschließlich Adultorganisation von Pygospio elegans Claparède, 1863 (Polychaeta, Spionidae). Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen
Schweigkofler M, Bartolomaeus T, von Salvini-Plawen L (1998) Ultrastructure and formation of hooded hooks in Capitella capitata (Capitellida, Annelida). Zoomorphology 118:117–128
Sensenbaugh T, Franzén A (1987) Fine structural observations of the apical organ in the larva of Polygordius (Annelida: Polychaeta). Scanning Microsc 1(1):181–189
Söderström A (1920) Studien über die Polychaetenfamilie Spionidae. Ph.D. Thesis, University of Uppsala, Uppsala
Storch V, Schlötzer-Schrehardt U (1988) Sensory structures. In: Westheide W, Hermans CO (eds) The ultrastructure of polychaeta. Microfauna marina, vol 4. Gustav Fischer Verlag, Stuttgart, New York
Tzetlin AB, Purschke G (2005) Pharynx and intestine. Hydrobiologia 535/536:199–225
Ushakov PV (1955) Polychaeta of the eastern sees of the U.S.S.R. Izdatel’stvo Adademii Nauk SSSR, Moscow
Verger-Bocquet M (1983) Etude infrastructurale des organes photorécepteurs chez les larves de Syllidens (Annélides, Polychètes). J Ultrastruct Res 84:67–72
von Salvini-Plawen L (1982) On the polyohyletic origin of photoreceptors. In: Westafall JA (ed) Visual cells in evolution. Raven Press, New York
von Salvini-Plawen L, Mayr E (1977) On the evolution of photoreceptors and eyes. Evol Biol 10:207–263
Westheide W (1985) The systematic position of the Dinophilidae and the archiannelid problem. In: Morris C, George JD, Gibson R, Platt HM (eds) The origins and relationships of lower invertebrates. Clarendon Press, Oxford, pp 310–326
Whittle AC, Golding DW (1974) The fine structure of prostomial photoreceptors in Eulalia viridis (Polychaeta; Annelida). Cell Tiss Res 154:379–398
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hausen, H. Ultrastructure of presumptive light sensitive ciliary organs in larvae of Poecilochaetidae, Trochochaetidae, Spionidae, Magelonidae (Annelida) and its phylogenetic significance. Zoomorphology 126, 185–201 (2007). https://doi.org/10.1007/s00435-007-0040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-007-0040-6