Abstract
Dysfunction and degeneration of synapses is a common feature of amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD). A GGGGCC hexanucleotide repeat expansion in the C9ORF72 gene is the main genetic cause of ALS/FTD (C9ALS/FTD). The repeat expansion leads to reduced expression of the C9orf72 protein. How C9orf72 haploinsufficiency contributes to disease has not been resolved. Here we identify the synapsin family of synaptic vesicle proteins, the most abundant group of synaptic phosphoproteins, as novel interactors of C9orf72 at synapses and show that C9orf72 plays a cell-autonomous role in the regulation of excitatory synapses. We mapped the interaction of C9orf72 and synapsin to the N-terminal longin domain of C9orf72 and the conserved C domain of synapsin, and show interaction of the endogenous proteins in synapses. Functionally, C9orf72 deficiency reduced the number of excitatory synapses and decreased synapsin levels at remaining synapses in vitro in hippocampal neuron cultures and in vivo in the hippocampal mossy fibre system of C9orf72 knockout mice. Consistent with synaptic dysfunction, electrophysiological recordings identified impaired excitatory neurotransmission and network function in hippocampal neuron cultures with reduced C9orf72 expression, which correlated with a severe depletion of synaptic vesicles from excitatory synapses in the hippocampus of C9orf72 knockout mice. Finally, neuropathological analysis of post-mortem sections of C9ALS/FTD patient hippocampus with C9orf72 haploinsufficiency revealed a marked reduction in synapsin, indicating that disruption of the interaction between C9orf72 and synapsin may contribute to ALS/FTD pathobiology. Thus, our data show that C9orf72 plays a cell-autonomous role in the regulation of neurotransmission at excitatory synapses by interaction with synapsin and modulation of synaptic vesicle pools, and identify a novel role for C9orf72 haploinsufficiency in synaptic dysfunction in C9ALS/FTD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD) are two closely related late-onset neurodegenerative disorders that overlap genetically, pathologically and clinically. ALS is characterised by selective demise of upper and lower motor neurons leading to progressive paralysis, whereas FTD is typified by degeneration of prefrontal cortical neurons that commonly gives rise to cognitive and behavioural impairments. In addition, extra-motor and other prefrontal regions such as the hippocampus are now being implicated in the pathogenesis of ALS/FTD [60, 82]. A GGGGCC (G4C2) repeat expansion in intron 1 of the C9orf72 gene is the most common genetic cause of ALS/FTD (C9ALS/FTD) [17, 61]. The exact mechanisms by which the repeat expansion causes C9ALS/FTD are unknown, but there is evidence for both loss- and gain-of-function. Three non-exclusive mechanisms have been proposed, namely loss of C9orf72 function due to the G4C2 repeat expansion causing decreased C9orf72 expression (C9orf72 haploinsufficiency), RNA toxicity caused by sequestration of RNA-binding proteins to sense and anti-sense repeat RNA foci, and protein toxicity deriving from non-canonical repeat associated non-AUG (RAN) translation of sense and anti-sense repeat transcripts into dipeptide repeat (DPR) proteins (Reviewed in [5]).
Recent evidence suggests a dual-hit model in which C9orf72 haploinsufficiency synergises with repeat-dependent gain-of-function mechanisms. In vitro, loss of C9orf72 increased levels of DPR proteins and thereby exacerbated protein toxicity [8]. In vivo, inactivation of one or both endogenous C9orf72 alleles in mice expressing human transgenes carrying the repeat expansion caused elevated DPR accumulation which was accompanied by exacerbated cognitive deficits, glial activation and hippocampal neuron loss [87] and C9orf72 deficiency promoted motor deficits of a C9ALS/FTD BAC transgenic mouse model in a gene dosage-dependent manner [65]. Understanding the pathophysiological impact of C9orf72 haploinsufficiency is key to our understanding of disease progression in C9ALS/FTD.
Despite its role in disease progression, the physiological function of the C9orf72 protein still remains poorly understood. We and others have identified C9orf72 as a regulator of membrane trafficking in the autophagy pathway through interactions with Rab GTPases, the ULK1 autophagy initiation complex, and lysosomes [2, 3, 20, 34, 64, 74, 80, 81]. In addition, C9orf72 has been shown to regulate Arf family GTPases which may explain its reported role in actin dynamics and endosomal sorting [15, 20, 66, 67, 72]. Recent work showing expression of the C9orf72 protein in synapses suggests C9orf72 may serve a physiological function in synapses [21, 47]. Absence of C9orf72 protein was confirmed on immunoblots (Supplementary Fig. 1c, Online Resource) of protein lysates of C9orf72-WT and C9orf72-KO mouse brains as described previously [76]. Briefly, brains were powdered in liquid nitrogen, homogenised by sonication in ice-cold homogenization buffer (Tris HCl, pH 8, 50 mM, NaCl 150 mM, EGTA pH 8.5 mM, MgCl2 1.5 mM, Triton X-100 1%, anhydrous glycerol 10%, phosphatase and protease inhibitor cocktail; Roche) and centrifuged at 13,000 rpm for 15 min at 4 °C. Hippocampi were homogenised using a Teflon potter in ice-cold homogenization buffer (Tris HCl, pH 8, 50 mM, NaCl 150 mM, EGTA pH 8.5 mM, MgCl2 1.5 mM, Triton X-100 1%, anhydrous glycerol 10%, phosphatase and protease inhibitor cocktail; Roche), sonicated and centrifuged at 13,000 rpm for 15 min at 4 °C. Supernatants were collected and stored at − 80 °C.
Procedures involving animals and their care that were conducted at the Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy adhered to the Mario Negri Institute for Pharmacological Research IRCCS institutional guidelines, that comply with national (D.lgs 26/2014; Authorization n.493/2019-PR issued on July 4, 2019, by Ministry of Health) and Mario Negri Institutional regulations and Policies providing internal authorisation for persons conducting animal experiments (Quality Management System certificate—UNI EN ISO 9001:2008—reg. N° 6121), the NIH Guide for the Care and Use of Laboratory Animals (2011 edition) and EU directives and guidelines (EEC Council Directive 2010/63/UE). All animals were housed under specific pathogen-free (SPF) conditions at a temperature of 22 ± 1 °C, a relative humidity of 55 ± 10% and 12-h light/dark cycle, 5 per cage. Food (standard pellets) and water were supplied ad libitum.
Procedures involving animals performed at the University of Sheffield, Sheffield, UK, were conducted according to the Animal (Scientific Procedures) Act 1986, under a Project Licence reviewed and approved by the University of Sheffield Ethical Review Sub-Committee, and the UK Animal Procedures Committee (London, UK). The UK Home Office code of practice for the housing and care of animals used in scientific procedures was followed. Mice were bred and housed in an SPF environment using a 12 h light/dark cycle, and a standardised room temperature of 21 ˚C. Mice were fed 2018 rodent diet (Harlan, UK) and provided with water ad libitum.
Immunohistochemistry
12-week-old mice were deeply anaesthetized with ketamine hydrochloride (150 mg/kg) and medetomidine (2 mg/kg) followed by intracardiac perfusion with PBS 0.1 M (pH 7.4) followed by perfusion with 4% paraformaldehyde (PFA, Merck) in PBS 0.1 M. Brains were removed, post-fixed overnight in PBS 0.1 M with 4% PFA followed by dehydration in PBS with 30% sucrose and embedded in cryostat medium OCT (Sakura Finetek, UK Ltd., Thatcham, UK). Brains were frozen in N-pentane at − 45 °C for 3 min, stored at − 80 °C before 30 µm coronal sections were cut on a cryostat. Free floating sections were blocked and permeabilized in PBS 0.01 M with 10% goat serum and 0.2% Triton and incubated overnight at 4 °C with primary antibodies in antibody diluent (PBS 0.01 M with 1% goat serum and 0.2% Triton) before being washed with PBS 0.01 M and incubated for 1 h at room temperature with secondary antibodies in PBS 0.01 M with 1% goat serum. After washing with PBS 0.01 M, sections were mounted on glass slides with coverslips using FluorSaveTM (Calbiochem, VWR International Ltd., Lutterworth, UK). Z-stacks of images were acquired on an Olympus IX81 microscope with a confocal scan unit (FV500, Olympus). Z-stacks of co-stained sections were acquired using the sequential scanning mode on an A1 Nikon confocal running NIS Elements at 40X magnification. For nuclear staining, sections were stained with Hoechst 33258 (1:500, Invitrogen) and images were acquired using an Olympus virtual slide system VS110 at 40 × magnification with VS-ASW software (Olympus) for image processing.
Immunohistochemistry staining of human brain samples was carried out on formalin-fixed, paraffin-embedded blocks of hippocampus and middle frontal gyrus of ALS/FTD patients with hexanucleotide expansions of the C9orf72 gene, non-C9orf72 related FTD patients as well as neurologically healthy controls. These post-mortem tissues were donated to the Sheffield Brain Tissue Bank (SBTB) with the consent of the next of kin. The SBTB Management Board gave ethical approval for this study under the provision to act as a Research Tissue Bank as approved by the Scotland A Research Ethics Committee (Ref 19/SS/0029, IRAS project ID 261271). Human sample information (pathological diagnosis, age, sex and C9orf72 status) is provided in Table 2. Sections of paraffin-embedded tissue were subjected to heat induced epitope retrieval (pressure cooker) at pH 9. Immunohistochemistry was carried out using standard ABC techniques with diaminobenzidine (DAB) as chromogen (Vectastain Elite ABC-HRP Kits, ImmPACT® DAB EqV Peroxidase (HRP) Substrate, Vector Laboratories Inc., Burlingame, CA, USA) with antibodies to Syn1, SV2 or C9orf72. Stained slides were digitised using the Hamamatsu NanoZoomer XP, visualized by NDP.view2 digital pathology software (Hamamatsu Photonics). Regions of interest from CA3 and CA4 areas were selected for digital image analysis by a qualified neuropathologist (JRH). Analysis was performed blinded to the genotype.
Electron microscopy
12-week-old mice were deeply anaesthetized with ketamine hydrochloride (150 mg/kg) and medetomidine (2 mg/kg) followed by intracardiac perfusion with PBS 0.1 M followed by 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) and 4% PFA in PBS 0.1 M. Brains were removed, post-fixed overnight in 2.5% glutaraldehyde and 4% PFA in PBS 0.1 M and washed 3–4 times for 1 h at 4 °C. A ~ 1 mm3 block containing the hippocampus was micro-dissected out from each whole brain before being treated with osmium tetroxide (1% in 0.1 M PBS) for 30 min. Samples were dehydrated through an ascending series of ethanol and propylene oxide, before embedding in Durcupan resin. During dehydration, sections were treated with uranyl acetate (1% in 70% ethanol) for 40 min. Durcupan resin was polymerised for 48 h in an oven set to 56 °C. Smaller regions of interest containing the CA3 region of hippocampus were identified and then cut from Durcupan-embedded sections before being mounted on Durcupan blocks and cutting ultra-thin sections (~ 70 nm) on an ultracut microtome (Leica) with an Ultra 45 Diamond Knife (Diatome, Hatfield, PA, USA). Sections were collected on formvar-coated slot grids and stained with lead citrate before imaging on a JEOL transmission electron microscope equipped with a Gatan digital camera (Jeol UK Ltd., Welwyn Garden City, UK).
Image analysis
Image analysis was performed using ImageJ/Fiji (http://imagej.nih.gov/ij/) [63] and CellProfiler-4.1.3 software [52].
PLA fluorescence intensities in EGFP-positive HEK293 cells were measured and the corrected total cell fluorescence (CTCF, arbitrary units, a.u.) was calculated as integrated density—(cell area × mean background fluorescence) using Fiji (http://imagej.nih.gov/ij/).
PLA signal in hippocampal neurons were analysed by counting the number of epifluorescence PLA signal spots detected per image using the ‘find maxima’ routine included in Fiji. The number of spots detected was normalized to the total mean pixel grey value of the synaptophysin epifluorescence intensity of the same image (norm PLA). Synaptophysin staining accounts for variability in neuron culture density across coverslips.
Synapse densities and properties of pre- and postsynaptic staining in 4-colour confocal images of cultured hippocampal neurons were analysed using an automated analysis pipeline in CellProfiler-4.1.3 [52]. Briefly, the dendritic marker MAP2 was used to identify neurites after feature enhancement using the Tubeness method and exclusion of cell body staining. To quantify neurite length, we binarized and skeletonised the enhanced neurite images and measured the skeleton intensity. To detect synapses, images of post- and presynapse markers were smoothed, enhanced using a white tophat filter and thresholded. Of the detected postsynapses only those that mapped to neurites were retained. Synapses were tallied when presynapses overlapped with postsynapses. For each image, measures of synapse density (synapse count/dendrite length) and mean values of pre- and postsynaptic parameters such as area size and intensity of pre- and postsynaptic markers of the identified synapses were exported to Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
To quantify immune staining of brain sections of C9orf72-WT and heterozygous and homozygous C9orf72-KO mice, confocal imaging stacks were analysed using Fiji and CellProfiler. Stacks were background subtracted and Z-projected (Sum of slices). For single stain images (Fig. 4), regions of interest (ROI) containing the mossy fibre area (hilus of the dentate gyrus (CA4) and the CA3/CA2 regions) were outlined manually by the operator while being blinded to the genotype. For higher magnification dual stain images (Fig. 5), regions of interest (ROI) containing hilus of the dentate gyrus (CA4) were selected. Projections and image masks created from the ROIs were imported in CellProfiler for analysis. For single stain images (Fig. 4) the area fraction and fluorescence intensity was determined. Positive signal within the masked mossy fibre area was determined by Robust Background thresholding (mean + 1 standard deviation). Fluorescence intensity was measured as the mean grey level of the thresholded area. For dual stained sections (Fig. 5), images of post- and presynapse markers were smoothed and enhanced using a white tophat filter and thresholded using the average threshold determined over all C9orf72-WT sections using the Robust Background thresholding method (mean + 1 standard deviation). Synapses were defined as postsynaptic spots that co-occurred with presynaptic spots. The number of synapses were normalised to the size of the region of interest to yield synaptic density.
On images of nuclear staining of the entire hippocampus, ROIs of the dentate gyrus were outlined manually and analysed blinded to the genotype to calculate the nuclei density (nuclei/mm2) of the dentate gyrus using Fiji.
To quantify DAB staining in C9ALS/FTD or FTD patient and healthy control brain sections, RGB images were analysed with Fiji using an automated colour threshold using the triangle method followed by particle analysis to determine the percentage of total image area positive for DAB staining. All analysis was performed blinded to the sample ID and disease status.
Individual synaptic profiles were identified in EM images based on the presence of clear pre- and postsynaptic elements, synaptic vesicles within the presynaptic nerve terminal, and a clear postsynaptic density [25, 38]. Synaptic vesicle counts were performed as described previously [24]. Briefly, vesicles were defined by the presence of a clear lumen, unbroken membranes, and a diameter of ∼ 50 nm. Pre-synaptic active zones were identified by their position opposite a postsynaptic density. Numbers of docked vesicles were estimated by measuring vesicles within a 125 nm radius of the presynaptic membrane. Analysis of TEM images was performed blind to the genotype of each sample.
Statistical analysis
Data are presented as bar charts plots with mean ± SEM or box and whisker plots where the box extends from the 25th to 75th percentiles and the whiskers show minimum and maximum; the line in the middle of the box is plotted at the median. All graphs were generated using Prism 9 software (GraphPad Software LLC., San Diego, CA, USA).
Calculations and statistical analysis were performed using Excel (Microsoft, Redmont, WA, USA) and Prism 9 software. Statistical significance between experimental groups was determined by one-way analyses of variance (ANOVA), paired or unpaired two-tailed t test, or one sample t and Wilcoxon tests, according to the data structure and distribution. Multiple comparisons following ANOVA were performed using "Tukey's multiple comparisons test". Sample sizes and further details can be found in the figure legends.
Results
C9orf72 interacts with synapsin
To identify novel interactors of C9orf72, we screened a human full-length cDNA yeast two-hybrid (Y2H) library containing approximately 15,000 ORF clones using the long isoform of human C9orf72 (C9orf72L) [17] as bait. The screen yielded 39 positive interacting clones. Thirty-four of these encoded full-length synapsin-3 (Syn3). The other 5 encoded another novel interacting protein. Therefore, this screen reproducibly revealed a strong interaction of C9orf72 with the presynaptic protein Syn3. Syn3 is a member of the synapsin protein family that in addition to Syn3 includes synapsin-1 (Syn1) and synapsin-2 (Syn2). The synapsin proteins are encoded by three distinct genes, SYN1, SYN2 and SYN3, and alternative splicing generates a and b isoforms of Syn1 and Syn2 and isoforms a-f of Syn3 [49]. Synapsins are peripheral membrane proteins found in all presynaptic terminals where they bind to synaptic vesicles and regulate their dynamics [70].
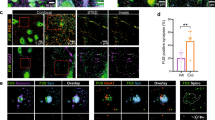
To confirm the interaction between C9orf72 and synapsin, we first performed co-immunoprecipitation assays of transfected proteins in HEK293 cells. In agreement with the Y2H interaction, V5-tagged Syn3a (V5-Syn3a) co-immunoprecipitated with Myc-tagged C9orf72L (Myc-C9orf72L) from V5-Syn3a and Myc-C9orf72L co-transfected cells but not from cells transfected with Myc-C9orf72L or V5-Syn3a alone (Fig. 1a). To determine whether the interaction between C9orf72 and synapsin was specific for the Syn3a isoform, we co-transfected HEK293 cells with Myc-C9orf72L and either empty EGFP vector, EGFP-tagged Syn1a or Syn3a, or YFP-tagged Syn2a. EGFP/YFP-synapsin was immunoprecipitated and the immune pellets were probed for EGFP/YFP and Myc. C9orf72 specifically co-immunoprecipitated with all three synapsin isoforms in these assays, albeit less efficiently with Syn2a (Fig. 1b).
Synapsin family proteins are novel binding partners of C9orf72. a Lysates of HEK293 cells co-transfected with Myc-C9orf72L (Myc-C9L) and either empty vector (EV) or V5-tagged Synapsin-3a (V5-Syn3a) were subjected to immunoprecipitation using an anti-Myc antibody. Immune pellets were probed for Myc-C9orf72L and V5-Syn3a on immunoblots. b Lysates of HEK293 cells co-transfected with Myc-C9L and either EV, EGFP or EGFP-Syn1a, YFP-Syn2a, EGFP-Syn3a were subjected to immunoprecipitation using an anti-GFP antibody. Immune pellets were probed for GFP/YFP and Myc-C9orf72L on immunoblots. c HEK293 cells were co-transfected with either Myc-C9L + V5-Syn3a or with Myc-C9L + V5-Syn1a. Transfections were laced with EGFP (green) to identify transfected cells. Cells were fixed and immunostained with both anti-V5 and anti-Myc antibodies and processed for PLA (PLA, magenta), nuclear staining with Hoechst (blue). Images are representative of the individual channels and their overlay. Scale bar 10 μm. Intensity of PLA signals per cell was analysed as corrected total cellular fluorescence (CTCF); data are presented as mean ± SEM; n (cells analysed) EV = 107, C9L = 92, Syn3a = 113, Syn1a = 79, C9L + Syn3a = 129, C9L + Syn1a = 109 from three or 4 replicate experiments. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001. Images showing the single transfection controls are shown in Supplementary Fig. 2, Online Resource. d Lysates of synaptosomes prepared from 12-week-old mouse brains were subjected to immunoprecipitation using an anti-C9orf72 antibody or an irrelevant antibody (Ctrl) raised in the same species as the C9orf72 antibody. Immune pellets were probed for endogenous SMCR8, C9orf72, Syn3 and Syn1 (* indicates a nonspecific band; enh, enhanced). e Lysates of synaptosomes prepared from 12-week-old C9orf72-WT and C9orf72-KO mouse brains were subjected to immunoprecipitation using an anti-C9orf72 antibody or a control antibody (M2). Immune pellets were probed for endogenous SMCR8, C9orf72, Syn3 and Syn1 (* indicates a nonspecific band; enh, enhanced). f 12DIV primary rat hippocampal neurons were fixed and immunostained with pairs of antibodies against C9orf72 and Syn3 (C9 + Syn3) or C9orf72 and Syn1 (C9 + Syn1) together with an antibody against synaptophysin and processed for PLA. Overlay PLA signals (red) between C9 + Syn3 or C9 + Syn1 and synaptophysin (white). Scale bar 5 μm. For quantification, the number of PLA spots per image was normalised (norm PLA) to the mean grey intensity of the synaptophysin image. Data are presented as mean ± SEM; n (images analysed) C9 = 14, Syn1 = 14, Syn3 = 15, C9 + Syn1 = 59, C9 + Syn3 = 62 from two or three replicate experiments. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001. Single antibody controls are shown in Supplementary Fig. 2, Online Resource
C9orf72 is known to form a complex with SMCR8 [64]. Since there is no SMCR8 ortholog in yeast, the Y2H data above show that interaction of C9orf72 and Syn3 does not require SMCR8. To further investigate a possible role of SMCR8 in the interaction of C9orf72 with synapsin we immunoprecipitated transfected SMCR8 carrying an mycDDK tag (SMCR8-mycDDK) from CRISPR/Cas9 C9orf72 knockout (KO) HEK293 cells (Supplementary Fig. 2a, Online Resource; Supplementary Materials and Methods, Online Resource) and probed the immune pellet for co-transfected V5-Syn3a. SMCR8 did not co-immunoprecipitate V5-Syn3a in absence of C9orf72. In contrast, when Myc-C9orf72L was co-transfected with SMCR8-mycDDK and V5-Syn3a, SMCR8 efficiently co-immunoprecipitated V5-Syn3a (Supplementary Fig. 2b, Online Resource). Hence synapsin interacts with the C9orf72/SMCR8 complex by interaction with C9orf72 but not SMCR8.
We next investigated the C9orf72/synapsin interaction in its cellular context using proximity ligation assays (PLA). Similar to conventional immunofluorescence, PLA uses pairs of primary antibodies that selectively recognise putative interacting proteins in fixed cells but the secondary antibodies used are linked to complementary oligonucleotide sequences (PLA probes) that generate a fluorescent ligation product when the two probes are in close proximity (< 40 nm); each signal corresponds to an interacting protein pair [69]. Using antibodies to the V5 and Myc tags, respectively, we observed proximity signals in all cells co-transfected with Myc-C9orf72L and V5-Syn1a or V5-Syn3a whereas very few signals were detected in empty vector or single transfected controls (transfections were laced with EGFP to identify transfected cells; Fig. 1c, Supplementary Fig. 3, Online Resource). Quantification of the background corrected PLA fluorescence signal showed that PLA intensities were significantly higher in cells co-expressing Myc-C9orf72L and V5-Syn3a or Myc-C9orf72L and V5-Syn1a compared to the controls (Fig. 1c, Supplementary Fig. 3, Online Resource). Thus, C9orf72 interacts with synapsin in situ.
Given the presynaptic localisation of C9orf72 and synapsin [21, 70] these data indicated that C9orf72 might interact with synapsin at synapses. Both synapsin and C9orf72 are highly expressed in the hippocampus, particularly within the mossy fibre system [21, 42, 53, 68, 3a, b). In contrast the decrease in SV2-positive synapses of 27.36 ± 8.155% was completely matched by a 23.37 ± 6.526% decrease in postsynapses (p = 0.2793, paired t test, n = 3 experiments, Fig. 3c). Thus, these data indicate that the reduction in the number of synapsin-positive synapses observed after C9orf72 knockdown is caused in part by a loss of excitatory synapses and in part by non-detection of synapses devoid of synapsin.
A recent report showed that C9orf72 deficiency led to enhanced microglia-mediated synaptic pruning and loss of synapses in the motor cortex of aged C9orf72-KO mice [43]. To investigate whether the changes in synapse density we observed in our cultures involved microglial pruning, we co-stained 12DIV hippocampal neuron cultures with a microglial marker, Iba1, a neuronal marker, beta-III tubulin, and a marker for non-neuronal cells, vimentin. No Iba1-positive microglia were observed in our hippocampal neuron cultures which are grown under conditions that are formulated specifically for neuronal cell requirements (Supplementary Fig. 7a, Online Resource). As a control we set up hippocampal cultures under specific conditions that support non-neuronal cells and confirmed that these contained Iba1-positive cells (Supplementary Fig. 7a, Online Resource). Thus, the reduction in the number of synapses observed after C9orf72 knockdown in our hippocampal neurons cultures is not caused by microglia-mediated elimination of synapses.
Changes in the ratio of functional excitatory and inhibitory synapses can affect the balance between excitatory and inhibitory neurotransmission. Disturbance of this equilibrium has emerged as a contributor to neurological disorders and neurodegenerative diseases, including ALS/FTD [23, 78, 83]. To determine if the effect of C9orf72 haploinsufficiency was specific for excitatory synapses, we labelled inhibitory synapses using antibodies to presynaptic Syn3 or the vesicular GABA transporter (VGAT) in combination with gephyrin, a postsynaptic scaffold in inhibitory glycinergic and GABAergic synapses [41]. In contrast to excitatory synapses, C9orf72 knockdown had no effect on the density of either Syn3- or VGAT-positive inhibitory synapses (Supplementary Fig. 7b, c, Online Resource). Similarly, there was no effect on the size of inhibitory presynapses (Supplementary Table 1, Online Resource) or levels of Syn3 in inhibitory presynapses, but there was a small reduction in VGAT levels (Supplementary Fig. 7b, c, Online Resource). Finally, knockdown of C9orf72 had no effect on the density of gephyrin-positive postsynapses (Supplementary Fig. 7b, c, Online Resource). Thus, C9orf72 haploinsufficiency selectively affects excitatory synapses.
Loss of C9orf72 impairs excitatory synapses in the hippocampus in vivo
The data presented above show that C9orf72 knockdown in dissociated hippocampal neuron cultures reduces the density of excitatory synapses. Furthermore, the levels of Syn1 and Syn3 were markedly reduced at excitatory synapses after knockdown of C9orf72 (Fig. 3). Even though embryonic hippocampal cultures are a well-characterised and commonly utilised model to study synapses, they lack the complexity and the tissue specific context of neurons within the intact brain. We therefore analysed the effects of loss of C9orf72 in the hippocampus of C9orf72-KO mice.
To investigate if loss of C9orf72 affected synapses in the hippocampus in vivo, we immunostained coronal sections of the dorsal hippocampus of 12-week-old C9orf72-WT, heterozygous C9orf72-KO (C9orf72-HET) and homozygous C9orf72-KO mice using antibodies to Syn1, Syn3 or the non-synapsin family presynaptic vesicle marker SV2. Staining for Syn1 and Syn3 was particularly prominent in the hippocampal mossy fibre system, namely the hilus area of the dentate gyrus (CA4) and the CA3/CA2 region (Fig. 4a–c), where also C9orf72 expression is pronounced [21, 42]. To assess if synapsin-positive synapses were affected by C9orf72 knockout we first determined the area fraction positive for Syn1 or Syn3 staining within this area of the hippocampus. For both Syn1 and Syn3, the area fraction of staining showed a significant, gene dosage-dependent reduction in C9orf72-HET and C9orf72-KO compared to C9orf72-WT controls (Fig. 4a, b). Quantification of protein levels by measuring the fluorescence intensity within this area showed a significant, gene dosage-dependent reduction in Syn3 levels in C9orf72-HET and C9orf72-KO (Fig. 4a, b). Syn1 levels were significantly reduced compared to C9orf72-WT controls but to the same extend in C9orf72-HET and C9orf72-KO. The reduction in synapsin levels was not due to a general effect on gene expression since immunoblot analysis of whole brain and hippocampal lysates did not show a difference between C9orf72-WT and C9orf72-KO mice (Supplementary Fig. 8a, Online Resource).
Loss of C9orf72 reduces levels of synapsin in the hippocampus in vivo. Representative images of hippocampal sections of 12-week-old C9orf72-WT, C9orf72-HET and C9orf72-KO mice stained for a Syn1, b Syn3 or c SV2. Syn1, Syn3, and SV2 levels in the mossy fibre area (outlined) were quantified as the area fraction of positive staining (Area Fraction) and the mean fluorescence intensity level (Intensity) within the outlined area was determined per section. Scale bar 100 µm. Data are presented as box and whisker plots; a Syn1, n (sections analysed) WT = 26, HET = 26, KO = 27 from 5 animals/genotype; b Syn3, n (sections analysed) WT = 30, HET = 28, KO = 26 from 5 animals/genotype; c SV2, n (sections analysed) WT = 26 HET = 24 and KO = 16 from 5 animals/genotype. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, ns (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
In a complementary approach, we co-stained hippocampal sections with Syn1/Homer or Syn3/PSD95 presynaptic/postsynaptic marker pairs and estimated synaptic density in the hilus of the dentate gyrus (CA4) by co-occurrence of the pre- and postsynaptic markers. C9orf72 again significantly reduced the number of Syn1 and Syn3-positive excitatory synapses in both C9orf72-HET and C9orf72-KO mice compared to the C9orf72-WT controls (Fig. 5a, b). Furthermore, the levels of Syn1 and Syn3 were significantly reduced in the remaining synapses in C9orf72-HET and C9orf72-KO mice (Supplementary Fig. 8b, Online Resource). Hence in agreement with dissociated hippocampal neurons, loss of C9orf72 resulted in a decrease in synapsin-positive synapses and reduced synapsin levels. To verify if the effects observed were specific for synapsin we analysed SV2 stained hippocampal sections. Loss of C9orf72 led to a significant, gene dosage-dependent reduction in the SV2-positive area fraction and fluorescence intensity in the C9orf72-HET and C9orf72-KO mice compared to C9orf72-WT (Fig. 4c). Similarly, analysis of synaptic density in the hilus of the dentate gyrus (CA4) after co-staining with SV2 and Homer showed a significant reduction in SV2/Homer positive synapses upon loss of C9orf72 (Fig. 5c), and decreased levels of SV2 in remaining synapses (Supplementary Fig. 8b, Online Resource).
Loss of C9orf72 reduces synaptic density in the hippocampus in vivo. Representative enhanced images showing a Syn1/Homer (green/magenta), b Syn3/PSD95 (green/magenta) or c SV2/Homer (green/magenta) pre- and postsynaptic marker pairs in the hilus of the dentate gyrus (CA4) region of the hippocampus of 12-week-old C9orf72-WT, C9orf72-HET and C9orf72-KO mice. Scale bar 20 µm. Synaptic density (density) was determined from the co-occurrence of pre- and postsynaptic marker pairs (white) in the hilus of the dentate gyrus (CA4) region. Data are presented as box and whisker plots; a Syn1/Homer, n (sections analysed) WT = 15, HET = 13, KO = 14 from 5 animals/genotype; b Syn3/PSD95, n (sections analysed) WT = 9, HET = 9, KO = 9 from 3 animals/genotype c SV2/Homer, n (sections analysed) WT = 15 HET = 14 and KO = 14 from 5 animals/genotype. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, ns (not significant), **P < 0.01, ****P < 0.0001
It is unlikely that neuron loss is responsible for the observed reductions in synapsin and SV2 staining because in agreement with previous reports [40, 87], we did not observe overt neuron loss in the hippocampus of the C9orf72-KO mice as analysed by determination of nuclei numbers in the dentate gyrus that innervates the mossy fibre area (Supplementary Fig. 8c, Online Resource). Hence, as was the case in vitro, loss of C9orf72 impairs hippocampal excitatory synapses in vivo.
Synapsin-positive structures are reduced in the hippocampus of C9ALS/FTD patients
We and others have reported hippocampal involvement in C9ALS/FTD, including p62, DPR and TDP-43 inclusions [1, 14, 35, 51, 54, 75], and occurrence of hippocampal sclerosis [55, 60].
The data above indicate that loss of C9orf72 affects excitatory synapses. Therefore, we next examined whether the synaptic changes observed in vitro in dissociated hippocampal neuron cultures and in vivo in C9orf72-HET and C9orf72-KO mice were also observed in the brains of C9ALS/FTD patients with C9orf72 haploinsufficiency by immunohistochemistry of post-mortem hippocampal sections (Fig. 6). Demographic, clinical and pathological characteristics are provided in Table 2.
Levels of C9orf72 and synapsin-1 are reduced in C9ALS/FTD hippocampus. a Representative image of post-mortem immunohistochemistry staining for C9orf72 in the hippocampus of a neurologically healthy control (CTRL) brain with the CA1, CA2, CA3 and CA4 region indicated. Scale bar: 1 mm. Box denotes zoom area. Open arrows in zoom illustrate the interface between the CA3 and CA2 region. Scale bar 100 µm. b Representative images of C9orf72 staining in the hippocampus of a CTRL and a C9ALS/FTD case. Insert: red areas depict the automated colour threshold used for quantification of the staining. Scale bar 50 µm. The area fraction of positive staining was determined in two CA3 and two CA4 regions per case and is presented as box and whisker plots; n (CA3/Ca4 regions), CTRL = 12 from 3 cases, C9ALS/FTD = 20 from 5 cases. Statistical significance was determined by unpaired two-tailed t test, *P < 0.05. c–d Representative images of Syn1 (c) and SV2 (d) staining in the hippocampus of a CTRL, C9ALS/FTD and FTD case. Insert: red areas depict the automated colour threshold used for quantification of the staining. Scale bar 50 µm. The area fraction of positive staining was determined in two CA3 and two CA4 regions per case and is presented as box and whisker plots; n (CA3/Ca4 regions); Syn1 n (CA3/Ca4 regions) CTRL = 16 from 4 individuals, C9ALS/FTD = 20 from 5 cases, FTD = 12 from three cases; SV2 n (CA3/Ca4 regions) CTRL = 12 from 4 individuals, C9ALS/FTD = 20 of 5 cases, FTD = 12 from three cases. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, ns (not significant), **P < 0.01, ***P < 0.001
We first labelled sections using C9orf72 antibodies to confirm haploinsufficiency. We found that the CA3 and CA4 subregions of the hippocampus had stronger C9orf72 signal compared to other subregions of Ammon’s horn and the dentate gyrus. This differential staining was particularly obvious at the interface between the CA2 and CA3 subregion (Fig. 6a, open arrows in zoom). In CA3 and CA4 the staining appeared as granular labelling of the neuropil, the synaptically dense areas between neuronal cell bodies consisting of unmyelinated axons, dendrites and glial cell processes. Besides labelling of the neuropil, C9orf72 antibodies also stained granules resembling synaptic boutons around the pyramidal cells of this region (Fig. 6a). The area fraction stained positive for C9orf72 was significantly reduced by approximately 28% in CA3/CA4 sections of C9ALS/FTD patients compared to healthy controls, demonstrating C9orf72 haploinsufficiency (Fig. 6b).
Similar as observed for C9orf72, both Syn1 and SV2 antibodies produced granular labelling of the neuropil and granular staining around cell bodies, consistent with labelling of synapses and was most prominent in CA3 and CA4 (Fig. 6c, d). Compared to healthy controls Syn1 staining was significantly reduced by approximately 36% in C9ALS/FTD cases (Fig. 6c) whereas there was no significant difference in SV2 staining between groups (Fig. 6d). Hence, the main impairment appears to be loss of synapsin from synapses rather than a general loss in synapses. To ascertain if this effect was specific for C9ALS/FTD cases we further evaluated Syn1 and SV2 staining in non-C9orf72-related FTD cases. There was no significant difference in Syn1 or SV2 staining between these FTD cases and neurologically normal controls (Fig. 6c, d). Thus, disruption to synapses in the hippocampus is a specific feature of C9ALS/FTD.
Loss of C9orf72 severely reduces the number of synaptic vesicles in excitatory synapses
The data above established that C9orf72 interacts with synapsin and that loss of C9orf72 causes depletion of synapsin from synapses and synapse loss (Figs. 1, 2, 3, 4, 5, 6). Synapsins are known to regulate neurotransmission by controlling synaptic vesicle pools and ultrastructural synaptic abnormalities have been observed in synapsin deficiency [11, 70]. Therefore, loss of C9orf72 and subsequent depletion of synapsin from synapses may disrupt synaptic vesicle pools. To explore this possibility, we performed high resolution morphological studies using transmission electron microscopy (TEM) on hippocampi from 12-week-old C9orf72-WT and C9orf72-KO mice. As expected, in C9orf72-WT mice excitatory synapses in the hippocampal CA3 region were densely packed with synaptic vesicles that were distributed in distinguishable reserve and docked synaptic vesicle pools (Fig. 7a). In marked contrast, C9orf72-KO synapses appeared severely depleted of synaptic vesicles (Fig. 7a). Quantification confirmed that the overall density of synaptic vesicles was reduced by approximately 30% in synaptic terminals of C9orf72-KO mice compared to C9orf72-WT (Fig. 7b). Moreover, the number of morphologically docked synaptic vesicles, synaptic vesicles that are located immediately adjacent to the presynaptic membrane in the active zone, was reduced by approximately 20% in C9orf72-KO mice (Fig. 7b). Taken together these results show that loss of C9orf72 and concomitant reductions in synapsin in synapses severely affects the number of synaptic vesicles and therefore synaptic vesicle pools in excitatory synapses.
Loss of C9orf72 affects synaptic vesicle pools in the hippocampus in vivo. a Representative transmission electron micrographs of the CA3 region of the hippocampus from 12-week old C9orf72-WT or C9orf72-KO mice. Scale bar 0.5 μm. Box denotes zoomed area. An excitatory nerve terminal is outlined by the red line in the zoomed area. Scale bar 0.15 μm. b Quantification of the density of synaptic vesicles (SV density, μm2) and the number of docked synaptic vesicles per synapse (Docked SVs/synapse) in C9orf72-WT and -KO excitatory synapses. Data are is presented as box and whisker plots; n (synapses analysed) WT = 81, KO = 105 obtained from 4 C9orf72-WT and 5 C9orf72-KO animals. Statistical significance was determined by unpaired two-tailed t test, **P < 0.01, ****P < 0.0001
C9orf72 haploinsufficiency affects excitatory neurotransmission and network function
Synapsin controls the storage and mobilisation of synaptic vesicles within the reserve pool and, furthermore, delivers synaptic vesicles to active zones and regulates neurotransmitter release and endocytosis [22, 26, 33, 70]. Given the depletion of synaptic vesicles from synapses in C9orf72-KO mice (Fig. 7) and loss of synapses and synapsin from remaining synapses in dissociated hippocampal neuron cultures treated with miRNA-C9 (Fig. 3), we hypothesised that C9orf72 haploinsufficiency may lead to decreased synaptic function and impaired neurotransmission.
To test this hypothesis, we performed whole-cell voltage-clamp experiments on 12-13DIV hippocampal neurons in which C9orf72 expression was selectively reduced by lentiviral miRNA-C9 delivery (Supplementary Fig. 9a, b, Online Resource). To investigate functional synaptic properties, we measured miniature postsynaptic currents. Because our data indicated a specific effect of C9orf72 haploinsufficiency on excitatory but not on inhibitory synapses (Figs. 3, 6, Supplementary Fig. 7, Online Resource), we conducted our recordings in the presence of the voltage-gated sodium channel blocker tetrodotoxin (TTX) together with blockers of inhibitory transmission, namely picrotoxin (PTX, GABAA receptor blocker) and strychnine (glycine receptor blocker), to isolate glutamatergic miniature excitatory postsynaptic currents (mEPSCs). mEPSC recordings in miRNA-C9 treated neurons revealed a substantial decrease in the frequency of mEPSC events by approximately 58% and a corresponding increase in the cumulative probability of the inter-mEPSC event interval compared to miRNA-NTC treated neurons (Fig. 8a, b). These results are consistent with a functional reduction in excitatory synaptic transmission in neurons haploinsuffient for C9orf72. Analysis of the individual mEPSC events showed that the postsynaptic current amplitude and decay kinetics were not significantly different in miRNA-NTC compared to miRNA-C9 treated neurons while the mEPSC rise time showed a modest increase when C9orf72 expression was reduced (Fig. 8c; Supplementary Fig. 9c, d, Online Resource). Because our experimental design favoured the isolation of AMPA receptor-mediated mEPSCs, these results indicate that the knockdown of C9orf72 did not impact upon the postsynaptic expression of glutamate-gated AMPA receptors. Thus, consistent with the reduction in excitatory synapses and loss of synapsin and synaptic vesicles in synapses (Figs. 3, 4, 5, 6, 7), the significant reduction of mEPSC frequency demonstrates that C9orf72 haploinsufficiency in hippocampal neurons affects the presynaptic compartment and functionally impairs excitatory neurotransmission.
C9orf72 haploinsufficiency affects excitatory neurotransmission and network function. a–c Primary rat hippocampal neurons were transduced with non-targeting control miRNA (miRNA NTC) or C9orf72 miRNA (miRNA C9orf72) lentivirus at 5DIV and miniature excitatory postsynaptic current (mEPSC) traces were recorded from single neurons on 12–13DIV at a holding potential of –70 mV (–84 mV with liquid junction potential correction). a Representative current traces, b mEPSC interevent interval time and c mEPSC amplitude data are presented as cumulative probability and mean ± SEM of n = number of cells recorded n (cells) miRNA-NTC = 15, miRNA-C9 = 13 from three individual batches of neurons. Statistical significance was determined by unpaired two-tailed t test, ns (not significant), *P < 0.05. d–g Multi-electrode array (MEA) recordings to measure network activity were performed at 12–13DIV on hippocampal neuron cultures transduced with non-targeting control miRNA (miRNA-NTC) or C9orf72 miRNA (miRNA-C9) lentivirus at 5DIV. d Representative traces of a single array channel electrode recorded from miRNA NTC or miRNA C9-transduced neurons. Scale bars 5 s, 50 μV. Network activity characteristics were quantified by determining the e interburst interval of network bursts, f intra-network burst spiking frequency, g network burst length. Data are presented as mean ± SEM of n = number of MEA arrays, n (arrays) miRNA-NTC = 6, miRNA-C9 = 7 from 4 individual batches of neurons. Statistical significance was determined by unpaired two-tailed t test, ns (not significant), *P < 0.05
In vitro cultured hippocampal neurons spontaneously develop functional network activity where action potentials (‘spikes’) become temporally organised into synchronized concentrated bursts. Synchronous bursts of network activity in these cultures rely on viable synaptic neurotransmission and network connectivity. Given the fact that we observed a substantially reduced frequency of synaptic mEPSC events in C9orf72-haploinsufficient neurons, we reasoned that this would impact upon neuron culture network activity.
To explore this, we cultured hippocampal neurons on multi-electrode arrays (MEA) that permit the detection of culture wide network activity and excitability. Network activity was measured on 12-13DIV hippocampal neurons in which C9orf72 expression was knocked down by treatment with lentiviral miRNA-C9 (Supplementary Fig. 9a, b, Online Resource) and assessed it according to a defined set of criteria (see Materials and Methods section).
We found that knockdown of C9orf72 increased the interval time between bursts (interburst interval) more than threefold, from 7.3 ± 2.1 s in miRNA-NTC to 22.6 ± 5.1 s in miRNA-C9-transduced neurons (Fig. 8d, e). C9orf72 haploinsufficiency did not affect the frequency of spikes within a burst or the actual duration of the burst (Fig. 8f, g). These data indicate that loss of C9orf72 reduced the basal network excitability of the cultures, which is in kee** with the reduction in excitatory synapses and loss of synapsin and synaptic vesicles in synapses we observed.
Discussion
C9orf72 haploinsufficiency is hypothesised to contribute to the disease phenotype in C9ALS/FTD, however the underlying mechanisms are unclear. Despite recent reports showing that C9orf72 is present in synapses [21, 87].
It is worth nothing that polymorphisms in UNC13A, which encodes the presynaptic protein Munc13-1, that have been identified as risk factors for ALS/FTD [18, 59] cause loss of Munc13-1 function [9, 50]. Munc13-1 primes synaptic vesicles for exocytosis and is essential for fusion of synaptic vesicles [4]. Consequently, similar to our observations in C9orf72 knockdown neurons, loss of Munc13-1 reduces excitatory synaptic transmission [4]. Thus, a pattern emerges in which dysfunction of excitatory synaptic transmission increases ALS/FTD disease risk and suggests that targeting synapse loss and synaptic dysfunction may be a viable strategy in ALS/FTD.
Our study demonstrates that C9orf72 directly interacts with the synapsin family of presynaptic vesicle proteins, impacting upon their levels in synapses and disturbing their role in modulating vesicle trafficking and neuronal function. These data support previous observations that excitatory synaptic dysfunction increases disease risk in ALS/FTD, and demonstrate for the first time that C9orf72 haploinsufficiency significantly contributes to altered synaptic density, regulation and function in C9ALS/FTD.
Finally, our study reveals a potentially deleterious neurological effect of reducing C9orf72 protein levels, which should be taken into consideration when designing anti-sense oligonucleotide therapeutics targeting C9orf72.
References
Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I et al (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122:691–702. https://doi.org/10.1007/s00401-011-0911-2
Amick J, Roczniak-Ferguson A, Ferguson SM (2016) C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol Biol Cell 27:3040–3051. https://doi.org/10.1091/mbc.E16-01-0003
Aoki Y, Manzano R, Lee Y, Dafinca R, Aoki M, Douglas AGL et al (2017) C9orf72 and RAB7L1 regulate vesicle trafficking in amyotrophic lateral sclerosis and frontotemporal dementia. Brain 140:887–897. https://doi.org/10.1093/brain/awx024
Augustin I, Rosenmund C, Südhof TC, Brose N (1999) Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400:457–461. https://doi.org/10.1038/22768
Balendra R, Isaacs AM (2018) C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat Rev Neurol 14:544–558. https://doi.org/10.1038/s41582-018-0047-2
Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M et al (2015) Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci USA 112:E2725–E2734. https://doi.org/10.1073/pnas.1504393112
Bigio EH, Weintraub S, Rademakers R, Baker M, Ahmadian SS, Rademaker A et al (2013) Frontotemporal lobar degeneration with TDP-43 proteinopathy and chromosome 9p repeat expansion in C9ORF72: clinicopathologic correlation. Neuropathology 33:122–133. https://doi.org/10.1111/j.1440-1789.2012.01332.x
Boivin M, Pfister V, Gaucherot A, Ruffenach F, Negroni L, Sellier C et al (2020) Reduced autophagy upon C9ORF72 loss synergizes with dipeptide repeat protein toxicity in G4C2 repeat expansion disorders. EMBO J 39:e100574. https://doi.org/10.15252/embj.2018100574
Brown A-L, Wilkins OG, Keuss MJ, Hill SE, Zanovello M, Lee WC et al (2022) TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 603:131–137. https://doi.org/10.1038/s41586-022-04436-3
Butti Z, Pan YE, Giacomotto J, Patten SA (2021) Reduced C9orf72 function leads to defective synaptic vesicle release and neuromuscular dysfunction in zebrafish. Commun Biol 4:792. https://doi.org/10.1038/s42003-021-02302-y
Cesca F, Baldelli P, Valtorta F, Benfenati F (2010) The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91:313–348. https://doi.org/10.1016/j.pneurobio.2010.04.006
Choi SY, Lopez-Gonzalez R, Krishnan G, Phillips HL, Li AN, Seeley WW et al (2019) C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat Neurosci 22:851–862. https://doi.org/10.1038/s41593-019-0397-0
Coleman WL, Bykhovskaia M (2010) Cooperative regulation of neurotransmitter release by Rab3a and synapsin II. Mol Cell Neurosci 44:190–200. https://doi.org/10.1016/j.mcn.2010.03.007
Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S et al (2012) Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 135:751–764. https://doi.org/10.1093/brain/awr365
Corrionero A, Horvitz HR (2018) A C9orf72 ALS/FTD ortholog acts in endolysosomal degradation and lysosomal homeostasis. Curr Biol 28:1522-1535.e5. https://doi.org/10.1016/j.cub.2018.03.063
Custer KL, Austin NS, Sullivan JM, Bajjalieh SM (2006) Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci 26:1303–1313. https://doi.org/10.1523/JNEUROSCI.2699-05.2006
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Diekstra FP, Van Deerlin VM, van Swieten JC, Al-Chalabi A, Ludolph AC, Weishaupt JH et al (2014) C9orf72 and UNC13A are shared risk loci for amyotrophic lateral sclerosis and frontotemporal dementia: a genome-wide meta-analysis. Ann Neurol 76:120–133. https://doi.org/10.1002/ana.24198
Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N (2014) Advanced methods of microscope control using μManager software. J Biol Methods 1:e10. https://doi.org/10.14440/jbm.2014.36
Farg MA, Sundaramoorthy V, Sultana JM, Yang S, Atkinson RA, Levina V et al (2014) C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum Mol Genet 23:3579–3595. https://doi.org/10.1093/hmg/ddu068
Frick P, Sellier C, Mackenzie IRA, Cheng CY, Tahraoui-Bories J, Martinat C et al (2018) Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers. Acta Neuropathol Commun 6:72. https://doi.org/10.1186/s40478-018-0579-0
Gerth F, Jäpel M, Pechstein A, Kochlamazashvili G, Lehmann M, Puchkov D et al (2017) Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc Natl Acad Sci USA 114:12057–12062. https://doi.org/10.1073/pnas.1715341114
Ghatak S, Talantova M, McKercher SR, Lipton SA (2021) Novel therapeutic approach for excitatory/inhibitory imbalance in neurodevelopmental and neurodegenerative diseases. Annu Rev Pharmacol Toxicol 61:701–721. https://doi.org/10.1146/annurev-pharmtox-032320-015420
Gillingwater TH, Ingham CA, Coleman MP, Ribchester RR (2003) Ultrastructural correlates of synapse withdrawal at axotomized neuromuscular junctions in mutant and transgenic mice expressing the Wld gene. J Anat 203:265–276. https://doi.org/10.1046/j.1469-7580.2003.00214.x
Gillingwater TH, Ingham CA, Parry KE, Wright AK, Haley JE, Wishart TM et al (2006) Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain 129:1546–1556. https://doi.org/10.1093/brain/awl101
Giovedì S, Darchen F, Valtorta F, Greengard P, Benfenati F (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. II. Functional effects of the Rab3A-synapsin I interaction. J Biol Chem 279:43769–43779. https://doi.org/10.1074/jbc.M404168200
Giovedì S, Vaccaro P, Valtorta F, Darchen F, Greengard P, Cesareni G et al (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. I. Identification and characterization of the synapsin I-Rab3 interactions in vitro and in intact nerve terminals. J Biol Chem 279:43760–43768. https://doi.org/10.1074/jbc.M403293200
Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC et al (2004) Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci 24:11368–11380. https://doi.org/10.1523/JNEUROSCI.3795-04.2004
Gray EG, Whittaker VP (1962) The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat 96:79–88
Henstridge CM, Pickett E, Spires-Jones TL (2016) Synaptic pathology: a shared mechanism in neurological disease. Ageing Res Rev 28:72–84. https://doi.org/10.1016/j.arr.2016.04.005
Henstridge CM, Sideris DI, Carroll E, Rotariu S, Salomonsson S, Tzioras M et al (2018) Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol 135:213–226. https://doi.org/10.1007/s00401-017-1797-4
Hilfiker S, Benfenati F, Doussau F, Nairn AC, Czernik AJ, Augustine GJ et al (2005) Structural domains involved in the regulation of transmitter release by synapsins. J Neurosci 25:2658–2669. https://doi.org/10.1523/JNEUROSCI.4278-04.2005
Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P (1999) Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354:269–279. https://doi.org/10.1098/rstb.1999.0378
Ho WY, Tai YK, Chang JC, Liang J, Tyan SH, Chen S et al (2019) The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15:827–842. https://doi.org/10.1080/15548627.2019.1569441
Hsiung GY, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E et al (2012) Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain 135:709–722. https://doi.org/10.1093/brain/awr354
Jensen BK, Schuldi MH, McAvoy K, Russell KA, Boehringer A, Curran BM et al (2020) Synaptic dysfunction induced by glycine-alanine dipeptides in C9orf72-ALS/FTD is rescued by SV2 replenishment. EMBO Mol Med 12:e10722. https://doi.org/10.15252/emmm.201910722
Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE et al (2007) New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci 27:4969–4977. https://doi.org/10.1523/JNEUROSCI.5561-06.2007
Koch S, Molchanova SM, Wright AK, Edwards A, Cooper JD, Taira T et al (2011) Morphologic and functional correlates of synaptic pathology in the cathepsin D knockout mouse model of congenital neuronal ceroid lipofuscinosis. J Neuropathol Exp Neurol 70:1089–1096. https://doi.org/10.1097/NEN.0b013e318238fc28
Koegl M, Uetz P (2007) Improving yeast two-hybrid screening systems. Brief Funct Genom Proteomic 6:302–312. https://doi.org/10.1093/bfgp/elm035
Koppers M, Blokhuis AM, Westeneng HJ, Terpstra ML, Zundel CA, Vieira de Sá R et al (2015) C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol 78:426–438. https://doi.org/10.1002/ana.24453
Krueger-Burg D, Papadopoulos T, Brose N (2017) Organizers of inhibitory synapses come of age. Curr Opin Neurobiol 45:66–77. https://doi.org/10.1016/j.conb.2017.04.003
Laflamme C, McKeever PM, Kumar R, Schwartz J, Kolahdouzan M, Chen CX et al (2019) Implementation of an antibody characterization procedure and application to the major ALS/FTD disease gene C9ORF72. Elife 8:e48363. https://doi.org/10.7554/eLife.48363
Lall D, Lorenzini I, Mota TA, Bell S, Mahan TE, Ulrich JD et al (2021) C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron 109:2275-2291.e8. https://doi.org/10.1016/j.neuron.2021.05.020
Leenders AG, Lopes da Silva FH, Ghijsen WE, Verhage M (2001) Rab3a is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Mol Biol Cell 12:3095–3102. https://doi.org/10.1091/mbc.12.10.3095
Levine TP, Daniels RD, Gatta AT, Wong LH, Hayes MJ (2013) The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics 29:499–503. https://doi.org/10.1093/bioinformatics/bts725
Lieberman OJ, Sulzer D (2020) The synaptic autophagy cycle. J Mol Biol 432:2589–2604. https://doi.org/10.1016/j.jmb.2019.12.028
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Livesey MR, Bilican B, Qiu J, Rzechorzek NM, Haghi G, Burr K et al (2014) Maturation of AMPAR composition and the GABAAR reversal potential in hPSC-derived cortical neurons. J Neurosci 34:4070–4075. https://doi.org/10.1523/JNEUROSCI.5410-13.2014
Longhena F, Faustini G, Brembati V, Pizzi M, Benfenati F, Bellucci A (2021) An updated reappraisal of synapsins: structure, function and role in neurological and psychiatric disorders. Neurosci Biobehav Rev 130:33–60. https://doi.org/10.1016/j.neubiorev.2021.08.011
Ma XR, Prudencio M, Koike Y, Vatsavayai SC, Kim G, Harbinski F et al (2022) TDP-43 represses cryptic exon inclusion in the FTD–ALS gene UNC13A. Nature 603:124–130. https://doi.org/10.1038/s41586-022-04424-7
Mackenzie IR, Arzberger T, Kremmer E, Troost D, Lorenzl S, Mori K et al (2013) Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol 126:859–879. https://doi.org/10.1007/s00401-013-1181-y
McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW et al (2018) Cell Profiler 3.0: next-generation image processing for biology. PLoS Biol 16:e2005970. https://doi.org/10.1371/journal.pbio.2005970
Melloni RH, Hemmendinger LM, Hamos JE, DeGennaro LJ (1993) Synapsin I gene expression in the adult rat brain with comparative analysis of mRNA and protein in the hippocampus. J Comp Neurol 327:507–520. https://doi.org/10.1002/cne.903270404
Murray ME, Bieniek KF, Banks Greenberg M, DeJesus-Hernandez M, Rutherford NJ, van Blitterswijk M et al (2013) Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol 126:545–554. https://doi.org/10.1007/s00401-013-1161-2
Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR et al (2011) Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122:673–690. https://doi.org/10.1007/s00401-011-0907-y
Pasniceanu IS, Atwal MS, Souza CDS, Ferraiuolo L, Livesey MR (2021) Emerging mechanisms underpinning neurophysiological impairments in C9ORF72 repeat expansion-mediated amyotrophic lateral sclerosis/frontotemporal dementia. Front Cell Neurosci 15:784833. https://doi.org/10.3389/fncel.2021.784833
Perkins EM, Burr K, Banerjee P, Mehta AR, Dando O, Selvaraj BT et al (2021) Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Mol Neurodegener 16:13. https://doi.org/10.1186/s13024-021-00433-8
Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P (1995) Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375:493–497. https://doi.org/10.1038/375493a0
Placek K, Baer GM, Elman L, McCluskey L, Hennessy L, Ferraro PM et al (2019) UNC13A polymorphism contributes to frontotemporal disease in sporadic amyotrophic lateral sclerosis. Neurobiol Aging 73:190–199. https://doi.org/10.1016/j.neurobiolaging.2018.09.031
Pletnikova O, Sloane KL, Renton AE, Traynor BJ, Crain BJ, Reid T et al (2014) Hippocampal sclerosis dementia with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 35:2419.e17–21. https://doi.org/10.1016/j.neurobiolaging.2014.04.009
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR et al (1995) Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375:488–493. https://doi.org/10.1038/375488a0
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Sellier C, Campanari ML, Julie Corbier C, Gaucherot A, Kolb-Cheynel I, Oulad-Abdelghani M et al (2016) Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J 35:1276–1297. https://doi.org/10.15252/embj.201593350
Shao Q, Liang C, Chang Q, Zhang W, Yang M, Chen JF (2019) C9orf72 deficiency promotes motor deficits of a C9ALS/FTD mouse model in a dose-dependent manner. Acta Neuropathol Commun 7:32. https://doi.org/10.1186/s40478-019-0685-7
Shi Y, Lin S, Staats KA, Li Y, Chang WH, Hung ST et al (2018) Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med 24:313–325. https://doi.org/10.1038/nm.4490
Sivadasan R, Hornburg D, Drepper C, Frank N, Jablonka S, Hansel A et al (2016) C9ORF72 interaction with cofilin modulates actin dynamics in motor neurons. Nat Neurosci 19:1610–1618. https://doi.org/10.1038/nn.4407
Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM et al (2012) Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135:693–708. https://doi.org/10.1093/brain/awr355
Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J et al (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3:995–1000. https://doi.org/10.1038/nmeth947
Song SH, Augustine GJ (2015) Synapsin isoforms and synaptic vesicle trafficking. Mol Cells 38:936–940. https://doi.org/10.14348/molcells.2015.0233
Starr A, Sattler R (2018) Synaptic dysfunction and altered excitability in C9ORF72 ALS/FTD. Brain Res 1693:98–108. https://doi.org/10.1016/j.brainres.2018.02.011
Su MY, Fromm SA, Zoncu R, Hurley JH (2020) Structure of the C9orf72 ARF GAP complex that is haploinsufficient in ALS and FTD. Nature 585:251–255. https://doi.org/10.1038/s41586-020-2633-x
Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T et al (1995) Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol 131:1789–1800. https://doi.org/10.1083/jcb.131.6.1789
Tang D, Sheng J, Xu L, Zhan X, Liu J, Jiang H et al (2020) Cryo-EM structure of C9ORF72-SMCR8-WDR41 reveals the role as a GAP for Rab8a and Rab11a. Proc Natl Acad Sci USA 117:9876–9883. https://doi.org/10.1073/pnas.2002110117
Troakes C, Maekawa S, Wijesekera L, Rogelj B, Siklós L, Bell C et al (2012) An MND/ALS phenotype associated with C9orf72 repeat expansion: abundant p62-positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathology 32:505–514. https://doi.org/10.1111/j.1440-1789.2011.01286.x
Trolese MC, Scarpa C, Melfi V, Fabbrizio P, Sironi F, Rossi M et al (2022) Boosting the peripheral immune response in the skeletal muscles improved motor function in ALS transgenic mice. Mol Ther. https://doi.org/10.1016/j.ymthe.2022.04.018
Vautrin J (2009) SV2 frustrating exocytosis at the semi-diffusor synapse. Synapse 63:319–338. https://doi.org/10.1002/syn.20610
Vucic S, Nicholson GA, Kiernan MC (2008) Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131:1540–1550. https://doi.org/10.1093/brain/awn071
Wang T, Liu H, Itoh K, Oh S, Zhao L, Murata D et al (2021) C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab 33:531-546.e9. https://doi.org/10.1016/j.cmet.2021.01.005
Webster CP, Smith EF, Bauer CS, Moller A, Hautbergue GM, Ferraiuolo L et al (2016) The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J 35:1656–1676. https://doi.org/10.15252/embj.201694401
Webster CP, Smith EF, Grierson AJ, De Vos KJ (2018) C9orf72 plays a central role in Rab GTPase-dependent regulation of autophagy. Small GTPases 9:399–408. https://doi.org/10.1080/21541248.2016.1240495
Westeneng HJ, Walhout R, Straathof M, Schmidt R, Hendrikse J, Veldink JH et al (2016) Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 87:1354–1360. https://doi.org/10.1136/jnnp-2016-313959
Williams KL, Fifita JA, Vucic S, Durnall JC, Kiernan MC, Blair IP et al (2013) Pathophysiological insights into ALS with C9ORF72 expansions. J Neurol Neurosurg Psychiatry 84:931–935. https://doi.org/10.1136/jnnp-2012-304529
**ao S, McKeever PM, Lau A, Robertson J (2019) Synaptic localization of C9orf72 regulates post-synaptic glutamate receptor 1 levels. Acta Neuropathol Commun 7:161. https://doi.org/10.1186/s40478-019-0812-5
Zhang D, Iyer LM, He F, Aravind L (2012) Discovery of novel DENN proteins: implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front Genet 3:283. https://doi.org/10.3389/fgene.2012.00283
Zhang M, Augustine GJ (2021) Synapsins and the synaptic vesicle reserve pool: floats or anchors. Cells 10:658. https://doi.org/10.3390/cells10030658
Zhu Q, Jiang J, Gendron TF, McAlonis-Downes M, Jiang L, Taylor A et al (2020) Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72. Nat Neurosci 23:615–624. https://doi.org/10.1038/s41593-020-0619-5
Acknowledgements
We thank Dr. Daniel Gitler, Ben-Gurion University, Israel for sharing synapsin reagents. We are grateful to the Sheffield Brain Tissue Bank and the very generous individuals who have donated tissue for this research and the next of kin who have accommodated this. The authors thank Steve Mitchell for technical assistance with electron microscopy and the technical and administrative team in SITraN for supporting our work. This work was funded by an Alzheimer’s Research UK project grant (ARUK-PG2019A-008 to KJDV) with additional support from the Medical Research Council (MRC) (MR/S025979/1 and MR/M013251/1 to KJDV) and by Regione Lombardia, Italy, “POR FESR 2014–2020 resources Call HUB Ricerca Innovazione” (CB, FS). EFS was supported by the Motor Neurone Disease Association (DEVOS/APR18/862-791 to KJDV and AJG). RNC was supported by a University of Sheffield Prize studentship. The Y2H screen was funded by the Thierry Latran Foundation (project RoCIP to KJDV and AJG).
Author information
Authors and Affiliations
Contributions
CSB, RNC, FS, MRL, THG, JRH, DJF, EFS, YBG, CPW, IC, AJG and KJDV performed and designed experiments. CSB, RNC, FS, MRL, THG, JRH, DJF, EFS, and KJDV analysed data. AJG generated key reagents for the study. CSB and KJDV wrote the manuscript with input and contributions from all authors. KJDV conceived the study. JRH, CB and KJDV supervised the research.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, C.S., Cohen, R.N., Sironi, F. et al. An interaction between synapsin and C9orf72 regulates excitatory synapses and is impaired in ALS/FTD. Acta Neuropathol 144, 437–464 (2022). https://doi.org/10.1007/s00401-022-02470-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02470-z