Abstract
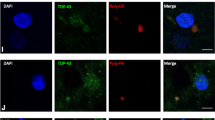
The most common cause of familial frontotemporal lobar degeneration with TAR DNA-binding protein-43 pathology (FTLD-TDP) has been found to be an expansion of a hexanucleotide repeat (GGGGCC) in a noncoding region of the gene C9ORF72. Hippocampal sclerosis (HpScl) is a common finding in FTLD-TDP. Our objective was to screen for the presence of C9ORF72 hexanucleotide repeat expansions in a pathologically confirmed cohort of “pure” hippocampal sclerosis cases (n = 33), outside the setting of FTLD-TDP and Alzheimer’s disease (AD). Using a recently described repeat-associated non-ATG (RAN) translation (C9RANT) antibody that was found to be highly specific for c9FTD/ALS, we identified a single “pure” HpScl autopsy case with a repeat expansion in C9ORF72 (c9HpScl). Mutation screening was also performed with repeat-primed polymerase chain reaction and further confirmed with Southern blotting. The c9HpScl patient had a 14-year history of a slowly progressive amnestic syndrome and a clinical diagnosis of probable AD. Neuropsychological testing revealed memory impairment, but no deficits in other cognitive domains. Autopsy showed hippocampal sclerosis with TDP-43 immunoreactive neuronal inclusions relatively limited to limbic lobe structures. Neuritic pathology immunoreactive for p62 was more frequent than TDP-43 in amygdala and hippocampus. Frequent p62-positive neuronal inclusions were present in cerebellar granule neurons as is typical of C9ORF72 mutation carriers. There was no significant FTLD or motor neuron disease. C9RANT was found to be sensitive and specific in this autopsy-confirmed series of HpScl cases. The findings in this patient suggest that the clinical and pathologic spectrum of C9ORF72 repeat expansion is wider than frontotemporal dementia and motor neuron disease, including cases of progressive amnestic dementia with restricted TDP-43 pathology associated with HpScl.




Similar content being viewed by others
References
Al-Sarraj S, King A, Troakes C et al (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122:691–702
Ala TA, Beh GO, Frey WH II (2000) Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology 54:843–848
Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW (2007) Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol 113:245–252
Ash PE, Bieniek KF, Gendron TF et al (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77:639–646
Baker M, Mackenzie IR, Pickering-Brown SM et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Benton AL, Hamsher K (1976) Multilingual Aphasia Examination. University of Iowa, Iowa
Bieniek KF, Murray ME, Rutherford NJ et al (2013) Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol 125:289–302
Boxer AL, Mackenzie IR, Boeve BF et al (2011) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82:196–203
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brettschneider J, Van Deerlin VM, Robinson JL et al (2012) Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol 123:825–839
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Dickson DW, Baker M, Rademakers R (2010) Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis 7:170–174
Dickson DW, Davies P, Bevona C et al (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (>or =80 years of age) humans. Acta Neuropathol 88:212–221
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gijselinck I, Engelborghs S, Maes G et al (2010) Identification of 2 loci at chromosomes 9 and 14 in a multiplex family with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol 67:606–616
Hodges J (2012) Familial frontotemporal dementia and amyotrophic lateral sclerosis associated with the C9ORF72 hexanucleotide repeat. Brain 135:652–655
Hyman BT, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13
Josephs KA, Jones AG, Dickson DW (2004) Hippocampal sclerosis and ubiquitin-positive inclusions in dementia lacking distinctive histopathology. Dement Geriatr Cogn Disord 17:342–345
Le Ber I, Camuzat A, Berger E et al (2009) Chromosome 9p-linked families with frontotemporal dementia associated with motor neuron disease. Neurology 72:1669–1676
Lee EB, Lee VM, Trojanowski JQ, Neumann M (2008) TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol 115:305–311
Lin WL, Castanedes-Casey M, Dickson DW (2009) Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 68:1167–1176
Luty AA, Kwok JB, Thompson EM et al (2008) Pedigree with frontotemporal lobar degeneration-motor neuron disease and Tar DNA binding protein-43 positive neuropathology: genetic linkage to chromosome 9. BMC Neurol 8:32
Mackenzie IR, Baker M, Pickering-Brown S et al (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113
Majounie E, Abramzon Y, Renton AE et al (2012) Repeat expansion in C9ORF72 in Alzheimer’s disease. N Engl J Med 366:283–284
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Momeni P, Schymick J, Jain S et al (2006) Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol 6:44
Montine TJ, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11
Mori K, Weng SM, Arzberger T et al (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339:1335–1338
Morita M, Al-Chalabi A, Andersen PM et al (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844
Murray ME, DeJesus-Hernandez M, Rutherford NJ et al (2011) Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122:673–690
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796
Murray ME, Senjem ML, Petersen RC et al (2010) Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol 67:1379–1385
Murray ME, Vemuri P, Preboske GM et al (2012) A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. J Neuropathol Exp Neurol 71:1113–1122
Nelson PT, Schmitt FA, Lin Y et al (2011) Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134:1506–1518
Pao WC, Dickson DW, Crook JE et al (2011) Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord 25:364–368
Rademakers R, Eriksen JL, Baker M et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642
Renton AE, Majounie E, Waite A et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268
Wojtas A, Heggeli KA, Finch N et al (2012) C9ORF72 repeat expansions and other FTD gene mutations in a clinical AD patient series from Mayo Clinic. Am J Neurodegener Dis 1:107–118
Zarow C, Sitzer TE, Chui HC (2008) Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep 8:363–370
Acknowledgments
We are grateful to all patients, family members, and caregivers who agreed to brain donation, without which these studies would have been impossible. We also acknowledge the expert technical assistance of Linda Rousseau and Virginia Phillips for histology, and Monica Castanedes-Casey for immunohistochemistry. We would like to thank Martha Purdy, John Gonzalez, and Beth Marten for brain bank coordination. Additionally, we appreciate the council of John A. Lucas for his recommendation of normative percentiles for neuropsychological scores. This research was funded by Mayo Foundation (Jacoby Professorship of Alzheimer Research); National Institutes of Health (P50-AG16574, P50-NS72187, P01-AG03949, R01-AG37491, R01-NS080882 and R01-AG026251); the ALS Therapy Alliance; and the State of Florida Alzheimer Disease Initiative. MEM is supported by a fellowship from the Robert and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Conflict of interest
Dr. Rademakers and Mrs. Dejesus-Hernandez have a patent on the clinical testing and therapeutic intervention for the hexanucleotide repeat expansion of C9ORF72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murray, M.E., Bieniek, K.F., Banks Greenberg, M. et al. Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72 . Acta Neuropathol 126, 545–554 (2013). https://doi.org/10.1007/s00401-013-1161-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1161-2