Abstract
Background
A four-drug cytochrome P450 (CYP) phenoty** cocktail was developed to rapidly and safely determine CYP2D6, CYP2C19, CYP2C9 and CYP1A2 enzyme activity and phenotype.
Methods
The cocktail consisted of the single CYP phenoty** probes of 50 mg tramadol (CYP2D6), 20 mg omeprazole (CYP2C19), 25 mg losartan (CYP2C9) and 200 mg caffeine (CYP1A2) and was administered as a single oral dose. For enzyme activity measurements, urine was collected as 8 h post-administration and blood was sampled at 4 h. The enzyme activity was determined by metabolic ratios of molar concentrations of the drugs and their enzyme catalyzed metabolites and was correlated to the relevant genotypes.
Results
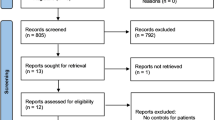
In a pilot study in 12 healthy male volunteers the CYP genotype–phenotype correlation and robustness of the cocktail was successfully determined without detection of any adverse drug reactions. In the subsequent population study, four female volunteers experienced unexpected and unacceptable moderate and severe adverse reactions (ARs) of headache, dizziness, nausea, vomiting, blue fingers, nails and lips and difficulties in urinating, which led to the study being prematurely terminated after inclusion of only 25 subjects (17 males, 7 females).
Conclusion
Attention must be paid to adverse reactions when designing new combinations of phenotype cocktails regardless of the doses and drugs involved. We specifically warn against the combination of tramadol, omeprazole, losartan and caffeine.
Similar content being viewed by others
References
Fuhr U, Jetter A, Kirchheiner J (2007) Appropriate phenoty** procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin Pharmacol Ther 81(2):270–283. doi:10.1038/sj.clpt.6100050
Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L (1995) Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics 5(6):358–363
Babaoglu MO, Yasar U, Sandberg M, Eliasson E, Dahl ML, Kayaalp SO, Bozkurt A (2004) CYP2C9 genetic variants and losartan oxidation in a Turkish population. Eur J Clin Pharmacol 60(5):337–342. doi:10.1007/S00228-004-0785-5
Fuhr U, Rost KL, Engelhardt R, Sachs M, Liermann D, Belloc C, Beaune P, Janezic S, Grant D, Meyer UA, Staib AH (1996) Evaluation of caffeine as a test drug for CYPIA2, NAT2 and CYP2E1 phenoty** in man by in vivo versus in vitro correlations. Pharmacogenetics 6(2):159–176. doi:10.1097/00008571-199604000-00003
Christensen M, Andersson K, Dalen P, Mirghani RA, Muirhead GJ, Nordmark A, Tybring G, Wahlberg A, Yasar U, Bertilsson L (2003) The Karolinska cocktail for phenoty** of five human cytochrome P450 enzymes. Clin Pharmacol Ther 73(6):517–528. doi:10.1016/S0009-9236(03)00050-X
Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, Shin JG (2007) Development of the "Inje cocktail'' for high-throughput evaluation of five human cytochrome p450 Isoforms in vivo. Clin Pharmacol Ther 82(5):531–540. doi:10.1038/Sj.Clpt.6100187
Noehr-Jensen L, Zwisler ST, Larsen F, Sindrup SH, Damkier P, Nielsen F, Brosen K (2009) Impact of CYP2C19 phenotypes on escitalopram metabolism and an evaluation of pupillometry as a serotonergic biomarker. Eur J Clin Pharmacol 65(9):887–894. doi:10.1007/s00228-009-0657-0
Rasmussen BB, Brix TH, Kyvik KO, Brosen K (2002) The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 12(6):473–478. doi:10.1097/00008571-200208000-00008
Rasmussen BB, Brosen K (1996) Determination of urinary metabolites of caffeine for the assessment of cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activity in humans. Ther Drug Monit 18(3):254–262. doi:10.1097/00007691-199606000-00006
Rasmussen BB, Brosen K (1997) Theophylline has no advantages over caffeine as a putative model drug for assessing CYP1A2 activity in humans. Brit J Clin Pharmacol 43(3):253–258. doi:10.1111/J.1365-2125.1997.00546.X
Damkier P, Brosen K (2000) Quinidine as a probe for CYP3A4 activity: Intrasubject variability and lack of correlation with probe-based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin Pharmacol Ther 68(2):199–209. doi:10.1067/Mcp.2000.108532
Pedersen RS, Damkier P, Brosen K (2005) Tramadol as a new probe for cytochrome P450 2D6 phenoty**: A population study. Clin Pharmacol Ther 77(6):458–467. doi:10.1016/J.Clpt.2005.01.014
Zwisler ST, Enggaard TP, Noehr-Jensen L, Pedersen RS, Mikkelsen S, Nielsen F, Brosen K, Sindrup SH (2009) The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin Pharmacol 104(4):335–344. doi:10.1111/J.1742-7843.2009.00378.X
Pedersen RS, Brosen K, Nielsen F (2003) Enantioselective HPLC method for quantitative determination of tramadol and O-desmethyltramadol in plasma and urine: Application to clinical studies. Chromatographia 57(5–6):279–285. doi:10.1007/Bf02492397
Nielsen AG, Damkier P, Pedersen RS, Brosen K (2009) Paroxetine is a dose dependent inhibitor of tramadol’s O-demethylation via Cyp2d6: pharmacokinetic and -dynamic consequences. Basic Clin Pharmacol 105:66–66
The Danish Ministry of Health and Prevention (2012) Notice on reporting of adverse reactions. BEK number 826 (01/08/2012) Available from: http://www.retsinfo.dk
Grünenthal. Tramadol hydrochloride, Summary of Product Characteristics. Grünenthal, Aachen, Germany. Last updated on the eMC: 18 Dec 2012
Merck Sharp & Dohme. Cozaar, Summary of Product Characteristics. Merck Sharp & Dohme, Whitehouse Station, NJ. Last updated on the eMC: 13 January 2012
AstraZeneca. Losec, Summary of Product Characteristics. AstraZeneca, London, UK. Last updated on the eMC: 21 December 2012
Aronson JJ (2006) Meyler's side effects of drugs. The International encyclopedia of adverse drug reactions and interactions. Elsevier, Amsterdam
Wu WN, McKown LA, Liao S (2002) Metabolism of the analgesic drug ULTRAM (tramadol hydrochloride) in humans: API-MS and MS/MS characterization of metabolites. Xenobiotica; the fate of foreign compounds in biological systems 32(5):411–425. doi:10.1080/00498250110113230
Grond S, Sablotzki A (2004) Clinical pharmacology of tramadol. Clin Pharmacokinet 43(13):879–923
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260(1):275–285
Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger W, Flores CM, Hertrampf T, Kogel B, Schiene K, Strassburger W, Terlinden R, Tzschentke TM (2012) Mechanistic and functional differentiation of tapentadol and tramadol. Expert Oin Pharmacother 13(10):1437–1449. doi:10.1517/14656566.2012.696097
Stoops WW, Lofwall MR, Nuzzo PA, Craig LB, Siegel AJ, Walsh SL (2012) Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology (Berl) 223(4):427–438. doi:10.1007/s00213-012-2739-4
Acknowledgments
This study was supported by grants from The Danish Research Council for Health and Disease (09–065539) and the Lundbeck Foundation (R32-A3119).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, R.S., Damkier, P., Christensen, M.M.H. et al. A cytochrome P450 phenoty** cocktail causing unexpected adverse reactions in female volunteers. Eur J Clin Pharmacol 69, 1997–1999 (2013). https://doi.org/10.1007/s00228-013-1561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1561-1