Abstract
A simple, efficient and rapid method for the synthesis of cephalosporin–protein conjugates was established. These conjugates were used as immunogens to produce monoclonal antibodies (mAbs) and as solid phase antigens in competitive indirect enzyme immunoassays (EIAs). With this generic approach, a novel set of monoclonal antibodies for cephalosporins was prepared, including ceftiofur and cephalexin as well as, reported here for the first time, cefoperazone, cefquinome and cephapirin. All 5 EIAs were highly sensitive, with standard curve IC50 values of 0.7 (ceftiofur), 1.1 (cefquinome), 5.2 (cephalexin), 13.8 (cefoperazone) and 40.3 ng mL−1 (cephapirin). Detection limits (IC30) ranged from 0.3 (ceftiofur mAb 1D7) to 17.2 ng mL−1 (cephapirin mAb 2F10). Specificity studies revealed that cephalosporin–antibody binding was strongly determined by the side chain residues of the cephem nucleus. Therefore all mAbs, to some extent, recognized other beta-lactam antibiotics with similar side chain residues. Within the group of cephalosporins approved for use in veterinary medicine, however, the final EIAs were highly selective for their respective antigen, except for the ceftiofur EIA which showed cross-reactions with cefquinome. The applicability of the five assays for drug residue testing in milk was demonstrated. In each EIA the target drug could be determined in milk with high accuracy and precision at concentrations far below the European Union maximum residue limits.

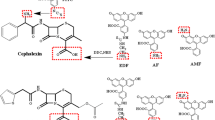
Structures of cephalosporins for which an MRL has been set within the EU. Monoclonal antibodies were produced against those substances shown in green lettering.



Similar content being viewed by others
Abbreviations
- 7-ACA:
-
7-aminocephalosporanic acid
- BSA:
-
bovine serum albumin
- EDC:
-
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide)
- EIA:
-
enzyme immunoassay
- EPB:
-
(R)-(−)-α-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]-4-hydroxybenzeneacetic acid
- EU:
-
European Union
- GlcOx:
-
glucose oxidase
- HRP:
-
horseradish peroxidise
- KLH:
-
keyhole limpet hemocyanin
- LOD:
-
limit of detection
- mAb:
-
monoclonal antibody
- MES:
-
2-(N-morpholino)ethanesulfonic acid hydrate
- MRL:
-
maximum residue limit
- NHS:
-
N-hydroxysuccinimide
- PBS:
-
phosphate buffered saline
References
Hornish RE, Kotarski SF (2002) Cephalosporins in veterinary medicine: ceftiofur use in food animals. Curr Top Med Chem 2:717–731
Rolinson GN, Geddes AM (2007) The 50th anniversary of the discovery of 6-aminopenicillanic acid (6-APA). Int J Antimicrob Agents 29:3–8
Mason IS, Kietzmann M (1999) Cephalosporins—pharmacological basis of clinical use in veterinary dermatology. Vet Dermatol 10:187–192
Collignon P, Aarestrup FM (2007) Extended-spectrum β-lactamases, food, and cephalosporin use in food animals. Clin Infect Dis 44:1391–1392
Mitchell JM, Griffiths MW, McEwen SA, McNab WB, Yee AJ (1998) Antimicrobial drug residues in milk and meat: causes, concerns, prevalence, regulations, tests, and test performance. J Food Prot 61:742–756
Boisseau J (1993) Basis for the evaluation of the microbiological risks due to veterinary drug residues in food. Vet Microbiol 35:187–192
Kirst E (2009) Rückstandsbestimmung in Kuhmilch gestern und heute. Prakt Tierarzt 90:356–362
Dewdney JM, Maes L, Raynaud JP, Blanc F, Scheid JP, Jackson T, Lens S, Verschueren C (1991) Risk assessment of antibiotic residues of [beta]-lactams and macrolides in food products with regard to their immuno-allergic potential. Food Chem Toxicol 29:477–483
Griffiths MW (2010) In: Griffiths MW (ed) Improving the safety and quality of milk, volume 1: milk production and processing, 1st edn. Cambridge, Woodhead Publ
Commission E (2010) Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Communities L 15:1–72
Holstege DM, Puschner B, Whitehead G, Galey FD (2002) Screening and mass spectral confirmation of beta-lactam antibiotic residues in milk using LC-MS/MS. J Agric Food Chem 50:406–411
Kantiani L, Farre M, Grases IFJM, Barcelo D (2010) Determination of antibacterials in animal feed by pressurized liquid extraction followed by online purification and liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem 398:1195–1205
Becker M, Zittlau E, Petz M (2004) Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 520:19–32
Bruno F, Curini R, di Corcia A, Nazzari M, Samperi R (2001) Solid-phase extraction followed by liquid chromatography-mass spectrometry for trace determination of beta-lactam antibiotics in bovine milk. J Agric Food Chem 49:3463–3470
Daeseleire E, De Ruyck H, Van Renterghem R (2000) Confirmatory assay for the simultaneous detection of penicillins and cephalosporins in milk using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 14:1404–1409
Cacciatore G, Petz M, Rachid S, Hakenbeck R, Bergwerff AA (2004) Development of an optical biosensor assay for detection of beta-lactam antibiotics in milk using the penicillin-binding protein 2x. Anal Chim Acta 520:105–115
Lamar J, Petz M (2007) Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal Chim Acta 586:296–303
Gustavsson E, Bjurling P, Degelaen J, Sternesjo A (2002) Analysis of beta-lactam antibiotics using a microbial receptor protein-based biosensor assay. Food Agric Immunol 14:121–131
Reybroeck W, Ooghe S, De Brabander HF, Daeseleire E (2010) Validation of the beta-s.t.a.r. 1 + 1 for rapid screening of residues of beta-lactam antibiotics in milk. Food Addit Contam Part A-Chem 27:1084–1095
Abouzied M, Sarzynski M, Walsh A, Wood H, Mozola M (2009) Validation study of a receptor-based lateral flow assay for detection of beta-lactam antibiotics in milk. J AOAC Int 92:959–974
Sorensen LK, Snor LK (2000) Determination of cephalosporins in raw bovine milk by high-performance liquid chromatography. J Chromatogr A 882:145–151
Kloth K, Rye-Johnsen M, Didier A, Dietrich R, Martlbauer E, Niessner R, Seidel M (2009) A regenerable immunochip for the rapid determination of 13 different antibiotics in raw milk. Analyst 134:1433–1439
Kachab EH, Wu WY, Chapman CB (1992) The development of an enzyme-linked immunosorbent assay (ELISA) for cephalexin. J Immunol Methods 147:33–41
**e H, Ma W, Liu L, Chen W, Peng C, Xu C, Wang L (2009) Development and validation of an immunochromatographic assay for rapid multi-residues detection of cephems in milk. Anal Chim Acta 634:129–133
Meyer UJ, Zhi ZL, Loomans E, Spener F, Meusel M (1999) Automated stand-alone flow injection immunoanalysis system for the determination of cephalexin in milk. Analyst 124:1605–1610
Meier B (2008) Entwicklung und Anwendung enzymimmunologischer Verfahren zum Nachweis von Cefalexin, Ceftiofur und Desfuroylceftiofur in Milch. Diss med vet. Justus-Liebig-Universität Gießen
Dillon PP, Daly SJ, Browne JG, Manning BM, Loomans E, Van Amerongen A, O’Kennedy R (2003) Application of an immunosensor for the detection of the beta-lactam antibiotic, cephalexin. Food Agric Immunol 15:225–234
Nagakura N, Shimizu T, Masuzawa T, Yanagihara Y (1990) Anti-cephalexin monoclonal antibodies and their cross-reactivities to cephems and penams. Int Arch Allergy Appl Immunol 93:126–132
Chen LB, Wang ZF, Ferreri M, Su JL, Han B (2009) Cephalexin residue detection in milk and beef by ELISA and colloidal gold based one-step strip assay. J Agric Food Chem 57:4674–4679
Rose BG, Buckley SA, KampsHoltzapple C, Beier RC, Stanker LH (1996) Ceftiofur sodium: monoclonal antibody development and cross-reactivity studies with structurally related cephalosporins. J Agric Food Chem 44:622–627
Thal J, Steffen M, Meier B, Schneider E, Adriany A, Usleber E (2011) Development of an enzyme immunoassay for the antibiotic cefquinome and its application for residue determination in cow’s milk after therapeutical mastitis treatment. Anal Bioanal Chem 399:1051–1059
Strasser A, Dietrich R, Usleber E, Märtlbauer E (2003) Immunochemical rapid test for multiresidue analysis of antimicrobial drugs in milk using monoclonal antibodies and hapten–glucose oxidase conjugates. Anal Chim Acta 495:11–19
Grabarek Z, Gergely J (1990) Zero-length crosslinking procedure with the use of active esters. Anal Biochem 185:131–135
Dietrich R, Usleber E, Martlbauer E (1998) The potential of monoclonal antibodies against ampicillin for the preparation of a multi-immunoaffinity chromatography for penicillins. Analyst 123:2749–2754
Farrell C, Rowell FJ, Dsilva C, Cumming RH (1994) Enzyme-linked-immunosorbent-assay for ceftazidime in airborne samples. Analyst 119:2411–2416
Rose BG, Kampsholtzapple C, Stanker LH (1995) Competitive indirect ELISA for ceftiofur sodium and the effect of different immunizing and coating antigen conjugates. Bioconjug Chem 6:529–535
Zhi ZL, Meyer UJ, Van den Bedem JW, Meusel M (2001) Evaluation of an automated and integrated flow-through immunoanalysis system for the rapid determination of cephalexin in raw milk. Anal Chim Acta 442:207–219
Trcka J, Seitz CS, Brocker EB, Gross GE, Trautmann A (2007) Aminopenicillin-induced exanthema allows treatment with certain cephalosporins or phenoxymethyl penicillin. J Antimicrob Chemother 60:107–111
Moats WA, Anderson KL, Rushing JE, Buckley S (2000) Conversion of cephapirin to deacetylcephapirin in milk and tissues of treated animals. J Agric Food Chem 48:498–502
Jaglan PS, Cox BL, Arnold TS, Kubicek MF, Stuart DJ, Gilbertson TJ (1990) Liquid chromatographic determination of desfuroylceftiofur metabolite of ceftiofur as residue in cattle plasma. JAOAC 73:26–30
Olson SC, Beconi-Barker MG, Smith EB, Martin RA, Vidmar TJ, Adams LD (1998) In vitro metabolism of ceftiofur in bovine tissues. J Vet Pharmacol Ther 21:112–120
Acknowledgements
This research project was partly supported by the Bavarian State Ministry of Nutrition, Agriculture and Forestry. We thank Ms. Brunhilde Minich, Ms. Franziska Witzko and Mr. Mostefa Djeffal for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Concentrations of immunoreagents used in the optimized competitive indirect EIAs for the determination of cephalosporins in buffer solution (DOCX 12 kb)
Table S2
Antiserum titres of mice immunized with cephalosporin-KLH conjugates (DOCX 11 kb)
Fig. S1
Standard curves for the detection of cefquinome using the four different mAbs in an indirect competitive EIA coated with cefquinome-NHS-BSA. The coefficients of variation for replicate standard concentrations were typically below 9.4% (DOCX 22 kb)
Fig. S2
Standard curves for the detection of cephalexin and cephapirin, using mAb 1F10 and 2F10, respectively, in an indirect competitive EIA coated with the accordant EDC conjugate. The coefficients of variation for replicate standard concentrations were typically below 6.0% for cephalexin coating and below 7.6% for cephapirin coating, respectively (DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Bremus, A., Dietrich, R., Dettmar, L. et al. A broadly applicable approach to prepare monoclonal anti-cephalosporin antibodies for immunochemical residue determination in milk. Anal Bioanal Chem 403, 503–515 (2012). https://doi.org/10.1007/s00216-012-5750-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5750-z