Abstract
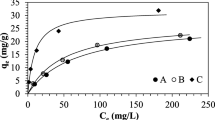
Simultaneous removal and recovery of cyanide and cadmium ions using a strong-base anion exchange resin was studied on the basis of formation of Cd-CN complexes at high pH in synthetic wastewater containing cyanide and cadmium ions. Strong-base anion exchange resin particles, of Dowex1X8-50, were contacted with synthetic aqueous solutions. For different molar ratios between cyanide and cadmium, ion exchange characteristics of cadmium-cyanide complexes were studied experimentally in a batch reactor. Treatment efficiencies of packed and fluidized beds were compared under various conditions. Several regenerants, NaSCN, NaCN, and NaOH, were used to regenerate the exhausted resin. The rates of regeneration and recovery for the various regenerants were estimated and discussed. The resin used in this work, Dowex1X8-50, can exchange about 6.6 CIST meq./g resin and 3.2 Cd2+ meq./g resin of cyanide and cadmium ions as complexes, respectively. Free cyanide ion has a lower selectivity than Cd-CN complexes on the anion exchange resin. The degree of treatment efficiency applied in this study was greater in the fluidized bed than packed bed. NaSCN was the best regenerant among regenerants used for regeneration of resin saturated with Cd-CN complexes.
Similar content being viewed by others
Abbreviations
- Co :
-
inlet concentration of reactant [mg/l]
- Ci :
-
effluent concentration of reactant [mg/l]
- Hi :
-
height of initial static bed [m]
- mM:
-
molar concentration [mg-mole/l]
- Tb :
-
breakthrough time [min]
- TCN :
-
total cadmium concentration [mg-mole/l]
- TCN :
-
total cyanide concentration [mg-mole/l]
- U:
-
superstitial fluid velocity in axial direction [mm/s]
- Umf :
-
minimum fluidizing velocity [mm/s]
References
Avery, N. L. and Fries, W., “Selective Removal of Cyanide from Industrial Waste Effluent with Ion-Exchange Resin,”Ind. Eng. Chem. Prod. Res. Dev.,14, 102 (1975).
Bhakta, D., Shukla, S. S. and Margrave, L. J., “A Novel Photocatalytic Method for Detoxification of Cyanide Wastes,”Environ. Sci. Technol.,26, 625(1992).
Cho, S. H. and Jeong, W. J., “Treatment of Electroplating Wastewater Containing Heavy Metal-Cyanide Complexes,”J. KSEE,1, 1096 (1999).
Goldblatt, E., “Recovery of Cyanide from Waste Cyanide Solutions by Ion Exchange,”Ind. Eng. Chem.,48, 2107 (1956).
Goncalves, M. M. M., Pinto, A. F. and Granato, M., “Biodegradation of Free Cyanide, Thiocyanide and Metal Complexed Cyanides in Solutions with Different Compositions,”Environmental Technology,19, 133 (1998).
Goto, M. and Goto, S., “Removal and Recovery of Heavy Metal by Ion Exchange Fiber,”J. Chem. Eng. Japan,20, 467 (1987).
Gupta, A., “Recovery of Metal-Cyanide Complexes from Electroplating Wastewaters by Ion Exchange,” Ph.D. Thesis, The Faculty of Princeton University, U.S.A. (1985).
Hassan, S. Q., Vitello, M. P. and Kupferle, M. J., “Treatment Technology Evaluation for Aqueous Metal and Cyanide,”J. Air Waste Manage. Assoc.,47, 719 (1991).
Herzorg, A. D., “Cyanide Leach Technology and Its Applicability to Alaskan Conditions,” Open File Report 89-90, U.S. Department of the Interior, Bureau of Mines (1990).
Horikawa, K. and Hirasawa, I., “Removal and Recovery of Nickel Ion from Wastewater of Electroless Plating by Reduction Crystallization,”Korean J. Chem. Eng.,17, 629 (2000).
Horner, J., “Cyanide Copper Plating,”Plat. Surf. Finish,82, 53 (1995).
Hsu, T. L., Tran., T. and Young, D., “Modeling of the Chemical Speciation of Cyanide Species-Application to Effluent Treatment,” Ausimm Extractive Metal Con., 133 (1991).
Kim, J. B., Sohn, J. E., Lee, S. S. and Lee, N. W., “Adsorption Characteristics of Cyanide Complex Anion of Heavy Metal on Activated Carbon,”HWAHAK KONGHAK,24, 1 (1986).
Kim, S. J., Hwang, K. R., Cho, S. Y. and Moon, H., “Simultaneous Removal of Cyanide and Copper Ions in a Semi-Fluidized Ion Exchanger Bed,” Proc. of the 6th Asian Conf. on Fluidized-Bed and Three-Phase Reactors, Cheju, Korea, 311 (1998).
Kim, S. J., Jeong, S. Y. and Cho, S. Y., “Removal and Recovery of Heavy Metal Ions in Fixed and Semi-Fluidized Beds,”Korean J. Chem. Eng.,15, 637 (1998).
Kurama, H. and Catalsarik, T., “Removal of Zinc Cyanide from a Leach Solution by an Anionic Ion-exchange Resin,”Desalination,129, 1 (2000).
Lee, H. S. and Suh, J. H., “Continuous Biosorption of Heavy Metal Ions by Ca-loaded Laminaria Japonica in Fixed Bed Column,”Korean J. Chem. Eng.,17, 477 (2000).
Lee, K. and Hong, J., “Separation and Recovery of Lead by Cation Exchange Process Combined with Precipitation,”AIChE J.,41, 2653 (1995).
Lukey, G. C., Van Deventer, J. and Shallcross, D. C., “Equilibrium Model for the Sorption of Gold Cyanide and Copper Cyanide on Trimethylamine Ion Exchange Resin in Saline Solution,”Hydrometallurgy,59, 101(2001).
Noh, B. I., Lee, C. W. and Yoon, T. K., “Parametric Studies on the Performance of Mixed-Bed Ion Exchange at Ultralow Concentrations — 1. Multicomponent System,”Korean J. Chem. Eng.,16, 737 (1999).
Park, C. K. and Hahn, P. S., “Reversibility and Linearity of Sorption for Some Cations onto a Bulguksa Granite,”Korean J. Chem. Eng.,16, 758 (1999).
Ritcey, G. M., “Tailing Management, Problems and Solutions in the Mining Industry,” Elsevier, N. Y., U.S.A. (1989).
Short, A. E., Haselmann, S. F. and Semmens, M. J., “The GM-IX Process-A Pilot Study for Recovering Zinc Cyanide,”J. Environmental Science and Health, Part A,32, 216 (1997).
Yu, Q. and Kaewsarn, P., “A Model for pH Dependent Equilibrium of Heavy Metal Biosorption,”Korean J. Chem. Eng.,16, 753 (1999).
Zhou, C. and Chin, D. T., “Copper Recovery and Cyanide Destruction with a Plating Barrel Cathode and a Packed-Bed Anode,”Plat. Surf. Finish,80, 69 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.J., Lim, K.H., Park, Y.G. et al. Simultaneous removal and recovery of cadmium and cyanide ions in synthetic wastewater by ion exchange. Korean J. Chem. Eng. 18, 686–691 (2001). https://doi.org/10.1007/BF02706387
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706387