Abstract
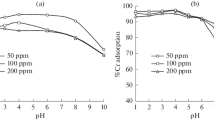
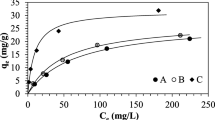
Ion exchange process is an alternative technique for removal of heavy metal ions from industrial wastewater. The main aim of this paper is to evaluate the performance of different ion exchange resins in removing Cr(VI) from wastewater. The effects of resin types and dosage, initial pH were examined systemically. The results showed that the performance of different resins had obvious difference for the removal of the Cr(VI) ions, in which the type of functional groups of the resin was the main factor. The SEM images indicated that the micro-morphology of resins before and after adsorption of the Cr(VI) presented a little difference. The EDS analysis showed that the adsorbed Cr(VI) was uniformly distributed at the surface of the resins with formation of oxygen-containing groups. The adsorption isotherms and kinetics of Cr(VI) by the different resins are also discussed.
Similar content being viewed by others
References
H. S. Altundogan, Process Biochem. 40, 1443 (2005).
S. Rengaraj, C. K. Joo, Y. Kim, and J. Yi, J. Hazard. Mater. 102, 257 (2003).
J. Nouri, A. H. Mahvi, G. R. Jahed, and A. A. Babae, Environ. Geol. 55, 1337 (2007).
J. Hu, I. Lo, and G. Chen, Sep. Purif. Technol. 56, 249 (2007).
S. Yalçin and R. Apak, Anal. Chim. Acta 505, 25 (2004).
W.-P. Yang, Z.-J. Zhang, and W. Deng, Anal. Chim. Acta. 485, 169 (2003).
G. M. Wuilloud, R. G. Wuilloud, J. C. A. de Wuilloud, and R. A. Olsina, J. Pharmaceut. Biomed. 31, 117 (2003).
V. Kumari, M. Sasidharan, and A. Bhaumik, Dalton Trans. 44, 1924 (2015).
F. A. Cotton and G. Wilkinson, Basic Inorganic Chemistry (Wiley, New York, 1976).
D. Clifford, A. K. Sengupta, and S. Subramonian, Water Res. 20, 1177 (1986).
A. K. Sengupta and D. Clifford, Environ. Sci. Technol. 20, 149 (1986).
C. Kim, Q. Zhou, B. Deng, E. C. Thornton, and H. Xu, Environ. Sci. Technol. 35, 2219 (2001).
M. Pettine, L. Campanella, and F. J. Millero, Environ. Sci. Technol. 36, 901 (2002).
S. Pamukcu, A. Weeks, and J. K. Wittle, Environ. Sci. Technol. 38, 1236 (2004).
S. T. Farrell and C. B. Breslin, Environ. Sci. Technol. 38, 4671 (2004).
T. Z. Sadyrbaeva, Chem. Eng. Process. 99, 183 (2016).
S. Rangaraj, K. H. Yeon, and S. H. Moon, J. Hazard. Mater. B 87, 273 (2001).
J. A. S. Tenorio and D. C. R. Espinosa, Waste Manage. 21, 637 (2001).
E. Korngold, N. Belayev, and L. Aronov, Sep. Purif. Technol. 33, 179 (2003).
L. Rafati, A. H. Mahvi, A. R. Asgari, and S. S. Hosseini, Int. J. Environ. Sci. Technol. 7, 147 (2010).
E. Pehlivan and S. Cetin, J. Hazard. Mater. 163, 448 (2009).
S. Edebali and E. Pehlivan, Powder Technol. 301, 520 (2016).
R. Hans, G. Senanayake, L. C. S. Dharmasiri, J. A. P. Mathes, and D. J. Kim, Hydrometallurgy 164, 208 (2016).
T. Engel, Physical Chemistry, 2nd ed. (Pearson, Prentice-Hall, 2006).
X. Qu, M. Tian, B. Liao, and A. Chen, Electrochim. Acta 55, 5367 (2010).
I. Langmuir, J. Am. Chem. Soc. 38, 2221 (1916).
H. M. F. Freundlich, J. Phys. Chem. 57, 385 (1906).
O. Kusku, B. L. Rivas, B. F. Urbano, M. Arda, N. Kabay, and M. Bryjak, J. Chem. Technol. Biotechnol. 89, 851 (2014).
W. Garcia-Vasquez, L. Dammak, C. Larchet, V. Nikonenko, and D. Grande, J. Membrane Sci. 507, 12 (2016).
K. **ao, F. Xu, L. Jiang, N. Duan, and S. Zheng, Chem. Eng. J. 283, 1349 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Li, X., Shi, S., Cao, H. et al. Comparative Study of Chromium(VI) Removal from Simulated Industrial Wastewater with Ion Exchange Resins. Russ. J. Phys. Chem. 92, 1229–1236 (2018). https://doi.org/10.1134/S0036024418060237
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418060237