Abstract
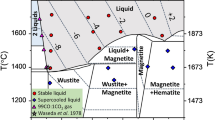
MgO containing ⩽5000 wt ppm Fe was heat-treated in various ways, so that the Fe was present in solution as Fe2+ or Fe3+, as precipitates of MgO.Fe2O3, or as metallic bcc Fe. Crystals were studied by optical absorption spectroscopy and microscopy. Heating in air at 1400‡ C converts most of the Fe to Fe3+ and some of this is associated with vacancies, particularly at high impurity levels. In crystals containing Fe3+, the magnitude of the hardening is relatively about four times less than that in alkali halides containing divalent metallic impurities, where all the impurities are associated with charge-compensating vacancies. Greater hardening is obtained when precipitates of MgO.Fe2O3 are present. Precipitates of metallic bcc Fe are formed on heating in hydrogen at temperatures >1000‡ C; these have the orientation relationships (001)MgO ∥ (001)Fe and [110]MgO ∥ [100]Fe.
Similar content being viewed by others
References
D. Woodhouse andJ. White,Trans. Brit. Ceram. Soc. 54 (1955) 333.
N. L. Bowen andJ. F. Schairer,Amer. J. Sci. 29 (1935) 153.
B. Phillips, S. Somiya, andA. Muan,J. Amer. Ceram. Soc. 44 (1961) 169.
G. W. Groves andM. E. Fine,J. Appl. Phys. 35 (1964) 3587.
J. E. Wertz, G. S. Saville, L. Hall, andP. Auzins,Proc. Brit. Ceram. Soc. 1 (1964) 59.
J. Brynestad andH. Flood,Z. Elecktrochem. 62 (1958) 953.
R. W. Soshea, A. J. Dekker, andJ. P. Sturtz,J. Phys. Chem. Solids 5 (1958) 23.
Y. Chen andW. A. Sibley,Phys. Rev. 154 (1967) 842.
B. Henderson, Phys. Dept., Keele Univ., Staffs., private communication.
D. L. Dexter,Sol. State Phys. 6 (1958) 355.
G. D. Miles, F. J. P. Clarke, B. Henderson, andR. D. King,Proc. Brit. Ceram. Soc. 6 (1966) 325.
W. G. Johnston andJ. J. Gilman,J. Appl. Phys. 30 (1959) 129.
R. L. Fleischer,Acta Met. 10 (1962) 835.
Idem, J. Appl. Phys. 33 (1962) 3504.
R. P. Harrison, P. L. Pratt, andC. W. A. Newey,Proc. Brit. Ceram. Soc. 1 (1964) 197.
W. G. Johnston,J. Appl. Phys. 33 (1962) 2050.
R. J. Stokes,J. Amer. Ceram. Soc. 48 (1965) 60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidge, R.W. The distribution of iron impurity in single-crystal magnesium oxide and some effects on mechanical properties. J Mater Sci 2, 339–346 (1967). https://doi.org/10.1007/BF00572417
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00572417