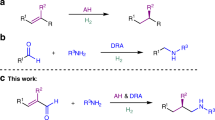
Abstract
The direct addition of amine N–H bonds across an unsaturated carbon–carbon linkage gives fast and highly atom-economical access to amines. This review provides an overview of the most effective stereoselective methods using various catalyst systems based on alkali, alkaline earth, and transition metals, as well as enzymatic and organocatalytic approaches. Except for a few sporadic examples in the last century, this field has evolved primarily over the last decade. Catalyst systems have reached enantioselectivities exceeding 90% ee for many substrate classes, but significant challenges remain, in particular in the stereoselective intermolecular hydroamination of unactivated alkenes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- Ar:
-
Aryl
- BDPP:
-
2,4-Bis(diphenylphosphino)pentane
- BINAP:
-
2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl
- Bn:
-
Benzyl
- Boc:
-
tert-Butoxycarbonyl
- Bu:
-
Butyl
- cat:
-
Catalyst
- Cbz:
-
Benzyloxycarbonyl
- COD:
-
Cyclooctadiene
- COE:
-
Cyclooctene
- Cp*:
-
Pentamethylcyclopentadienyl
- Cy:
-
Cyclohexyl
- d:
-
Day(s)
- DCE:
-
1,2-Dichloroethane
- DCM:
-
Dichloromethane
- DIOP:
-
Bis(diphenylphosphino-methyl)-2,2-dimethyl-1,3-dioxolane
- DMF:
-
Dimethylformamide
- dr:
-
Diastereomer ratio
- ebthi:
-
Ethylenebis(tetrahydroindenyl)
- ee :
-
Enantiomer excess
- equiv:
-
Equivalent(s)
- Et:
-
Ethyl
- Fmoc:
-
Fluorenylmethoxycarbonyl
- Grubbs-I:
-
Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
- Grubbs-II:
-
Benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinyl-idene]dichloro(tricyclohexylphosphine)ruthenium
- h:
-
Hour(s)
- i-Pr:
-
Isopropyl
- KHMDS:
-
Potassium hexamethyldisilazide potassium bis(trimethylsilyl)amide
- LDA:
-
Lithium diisopropylamide
- Mbs:
-
p-Methoxy benzenesulfonyl
- Me:
-
Methyl
- MeO-BIPHEP:
-
6,6-Dimethoxy-2,2-bis(diphenylphosphino)-1,1-biphenyl
- Mes:
-
Mesityl 2,4,6-trimethylphenyl
- mol:
-
Mole(s)
- MS:
-
Molecular sieves
- Mts:
-
Mesitylenesulfonyl
- MW:
-
Microwave
- Nf:
-
Nonafluorobutanesulfonyl
- NMO:
-
N-Methylmorpholine N-oxide
- N t :
-
Turnover frequency
- PAL:
-
Phenylalanine ammonia lyase
- PG:
-
Protecting group
- Ph:
-
Phenyl
- PMB:
-
4-Methoxyphenyl
- PNB:
-
4-Nitrobenzoyl
- rt:
-
Room temperature
- SEGPHOS:
-
4,4′-Bi-1,3-benzodioxole-5,5′-diylbis(diphenylphosphane)
- TBDPS:
-
tert-Butyldiphenylsilyl
- TBS:
-
tert-Butyldimethylsilyl
- t-Bu:
-
tert-Butyl
- Tf:
-
Trifluoromethanesulfonyl (triflyl)
- TFAA:
-
Trifluoroacetic anhydride
- THF:
-
Tetrahydrofuran
- TiPP:
-
2,4,6-Triisopropyphenyl
- TIPS:
-
Triisopropylsilyl
- Tol:
-
4-Methylphenyl
- Troc:
-
2,2,2-Trichloroethoxycarbonyl
- Ts:
-
Tosyl 4-toluenesulfonyl
- Xylyl-BINAP:
-
2,2′-Bis[di(3,5-xylyl)phosphino]-1,1′-binaphthyl
References
Müller TE, Beller M (1998) Chem Rev 98:675
Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M (2008) Chem Rev 108:3795
Brunet JJ, Neibecker D (2001) In: Togni A, Grützmacher H (eds) Catalytic heterofunctionalization from hydroamination to hydrozirconation. Wiley-VCH, Weinheim, p 91
Doye S (2009) In: Schaumann E, Enders D (eds) Science of synthesis, vol 40a. Georg Thieme Verlag, Stuttgart, p 241
Reznichenko AL, Hultzsch KC (2013) Top Organomet Chem 43:51
Nishina N, Yamamoto Y (2013) Top Organomet Chem 43:115
Roesky PW, Müller TE (2003) Angew Chem Int Ed 42:2708
Hultzsch KC (2005) Adv Synth Catal 347:367
Hultzsch KC (2005) Org Biomol Chem 3:1819
Reznichenko AL, Hultzsch KC (2010) In: Nugent T (ed) Chiral amine synthesis: methods, developments and applications. Wiley-VCH, Weinheim, p 341
Reznichenko AL, Hultzsch KC (2010) In: De Vries JG, Molander GA, Evans PA (eds) Science of synthesis, stereoselective synthesis, vol 1. Georg Thieme Verlag, Stuttgart, p 689
Aillaud I, Collin J, Hannedouche J, Schulz E (2007) Dalton Trans 5105
Hannedouche J, Collin J, Trifonov A, Schulz E (2011) J Organomet Chem 696:255
Zi G (2009) Dalton Trans 9101
Zi G (2011) J Organomet Chem 696:68
Hannedouche J, Schulz E (2013) Chem Eur J 19:4972
Steinborn D, Taube R (1986) Z Chem 26:349
Taube R (2002) In: Cornils B, Herrmann WA (eds) Applied homogeneous catalysis with organometallic compounds, vol 1. Wiley-VCH, Weinheim, p 513
Li-Wen Xu C-GX (2005) Eur J Org Chem 633
Hii KK (2006) Pure Appl Chem 78:341
Zhang Z, Lee SD, Widenhoefer RA (2009) J Am Chem Soc 131:5372
Sevov CS, Zhou J, Hartwig JF (2012) J Am Chem Soc 134:11960
Reznichenko AL, Nguyen HN, Hultzsch KC (2010) Angew Chem Int Ed 49:8984
Reznichenko AL, Hultzsch KC (2013) Organometallics 32:1394
Kawatsura M, Hartwig JF (2000) J Am Chem Soc 122:9546
Li K, Horton PN, Hursthouse MB, Hii KK (2003) J Organomet Chem 665:250
Hu A, Ogasawara M, Sakamoto T, Okada A, Nakajima K, Takahashi T, Lin W (2006) Adv Synth Catal 348:2051
Nettekoven U, Hartwig JF (2002) J Am Chem Soc 124:1166
Utsunomiya M, Hartwig JF (2003) J Am Chem Soc 125:14286
Johns AM, Utsunomiya M, Incarvito CD, Hartwig JF (2006) J Am Chem Soc 128:1828
Besson L, Goré J, Cazes B (1995) Tetrahedron Lett 36:3857
Al-Masum M, Meguro M, Yamamoto Y (1997) Tetrahedron Lett 38:6071
Meguro M, Yamamoto Y (1998) Tetrahedron Lett 39:5421
Löber O, Kawatsura M, Hartwig JF (2001) J Am Chem Soc 123:4366
Minami T, Okamoto H, Ikeda S, Tanaka R, Ozawa F, Yoshifuji M (2001) Angew Chem Int Ed 40:4501
Pawlas J, Nakao Y, Kawatsura M, Hartwig JF (2002) J Am Chem Soc 124:3669
Sakai N, Ridder A, Hartwig JF (2006) J Am Chem Soc 128:8134
Landis CR, Halpern J (1987) J Am Chem Soc 109:1746
Dorta R, Egli P, Zürcher F, Togni A (1997) J Am Chem Soc 119:10857
Casalnuovo AL, Calabrese JC, Milstein D (1988) J Am Chem Soc 110:6738
Beller M, Breindl C, Eichberger M, Hartung CG, Seayad J, Thiel OR, Tillack A, Trauthwein H (2002) Synlett 1579
Zhou J, Hartwig JF (2008) J Am Chem Soc 130:12220
Pan S, Endo K, Shibata T (2012) Org Lett 14:780
Gröger H (2010) In: Ojima I (ed) Catalytic asymmetric synthesis. Wiley, Hoboken, p 269
Hyun MW, Yun YH, Kim JY, Kim SH (2011) Mycobiology 39:257
Hauer B, Schneider N, Drew D, Ditrich K, Turner N, Nestl BM (2011) (BASF Aktiengesellschaft, Germany). WO2011/012,632
Cooper NJ, Knight DW (2004) Tetrahedron 60:243
MacDonald MJ, Schipper DJ, Ng PJ, Moran J, Beauchemin AM (2011) J Am Chem Soc 133:20100
MacDonald MJ, Hesp CR, Schipper DJ, Pesant M, Beauchemin AM (2013) Chem Eur J 19:2597
Guimond N, MacDonald MJ, Lemieux V, Beauchemin AM (2012) J Am Chem Soc 134:16571
Hong S, Marks TJ (2004) Acc Chem Res 37:673
Reznichenko AL, Hultzsch KC (2010) Struct Bond 137:1
Gagné MR, Stern CL, Marks TJ (1992) J Am Chem Soc 114:275
Motta A, Lanza G, Fragalà IL, Marks TJ (2004) Organometallics 23:4097
Manna K, Kruse ML, Sadow AD (2011) ACS Catal 1:1637
Tobisch S (2011) Chem Eur J 17:14974
Tobisch S (2012) Dalton Trans 41:9182
Gagné MR, Brard L, Conticello VP, Giardello MA, Stern CL, Marks TJ (1992) Organometallics 11:2003
Giardello MA, Conticello VP, Brard L, Gagné MR, Marks TJ (1994) J Am Chem Soc 116:10241
Kirby JA (1981) Adv Phys Org Chem 17:183
Jung ME, Piizzi G (2005) Chem Rev 105:1735
Douglass MR, Ogasawara M, Hong S, Metz MV, Marks TJ (2002) Organometallics 21:283
Ryu J-S, Marks TJ, McDonald FE (2004) J Org Chem 69:1038
Vitanova DV, Hampel F, Hultzsch KC (2007) J Organomet Chem 692:4690
Molander GA, Dowdy ED (1998) J Org Chem 63:8983
Molander GA, Dowdy ED (1999) J Org Chem 64:6515
Ryu J-S, Marks TJ, McDonald FE (2001) Org Lett 3:3091
Kim YK, Livinghouse T (2002) Angew Chem Int Ed 41:3645
Chapurina Y, Hannedouche J, Collin J, Guillot R, Schulz E, Trifonov A (2010) Chem Commun 46:6918
Chapurina Y, Ibrahim H, Guillot R, Kolodziej E, Collin J, Trifonov A, Schulz E, Hannedouche J (2011) J Org Chem 76:10163
Hultzsch KC, Gribkov DV, Hampel F (2005) J Organomet Chem 690:4441
O’Shaughnessy PN, Scott P (2003) Tetrahedron Asymmetry 14:1979
O’Shaughnessy PN, Knight PD, Morton C, Gillespie KM, Scott P (2003) Chem Commun 1770
O’Shaughnessy PN, Gillespie KM, Knight PD, Munslow I, Scott P (2004) Dalton Trans 2251
Hong S, Tian S, Metz MV, Marks TJ (2003) J Am Chem Soc 125:14768
Gribkov DV, Hultzsch KC, Hampel F (2003) Chem Eur J 9:4796
Gribkov DV, Hultzsch KC (2004) Chem Commun 730
Gribkov DV, Hampel F, Hultzsch KC (2004) Eur J Inorg Chem 4091
Gribkov DV, Hultzsch KC, Hampel F (2006) J Am Chem Soc 128:3748
Collin J, Daran J-D, Schulz E, Trifonov A (2003) Chem Commun 3048
Collin J, Daran J-D, Jacquet O, Schulz E, Trifonov A (2005) Chem Eur J 11:3455
Riegert D, Collin J, Meddour A, Schulz E, Trifonov A (2006) J Org Chem 71:2514
Riegert D, Collin J, Daran J-D, Fillebeen T, Schulz E, Lyubov D, Fukin G, Trifonov A (2007) Eur J Inorg Chem 1159
Aillaud I, Wright K, Collin J, Schulz E, Mazaleyrat J-P (2008) Tetrahedron Asymmetry 19:82
Hannedouche J, Aillaud I, Collin J, Schulz E, Trifonov A (2008) Chem Commun 3552
Aillaud I, Lyubov D, Collin J, Guillot R, Hannedouche J, Schulz E, Trifonov A (2008) Organometallics 27:5929
Aillaud I, Collin J, Duhayon C, Guillot R, Lyubov D, Schulz E, Trifonov A (2008) Chem Eur J 14:2189
Aillaud I, Collin J, Hannedouche J, Schulz E, Trifonov A (2010) Tetrahedron Lett 51:4742
Queffelec C, Boeda F, Pouilhés A, Meddour A, Kouklovsky C, Hannedouche J, Collin J, Schulz E (2011) ChemCatChem 3:122
Aillaud I, Olier C, Chapurina Y, Collin J, Schulz E, Guillot R, Hannedouche J, Trifonov A (2011) Organometallics 30:3378
Chapurina Y, Guillot R, Lyubov D, Trifonov A, Hannedouche J, Schulz E (2013) Dalton Trans 42:507
Kim JY, Livinghouse T (2005) Org Lett 7:1737
Kim H, Kim YK, Shim JH, Kim M, Han M, Livinghouse T, Lee PH (2006) Adv Synth Catal 348:2609
Lovick HM, An DK, Livinghouse TS (2011) Dalton Trans 40:7697
Meyer N, Zulys A, Roesky PW (2006) Organometallics 25:4179
Yu X, Marks TJ (2007) Organometallics 26:365
Heck R, Schulz E, Collin J, Carpentier J-F (2007) J Mol Cat A 268:163
**ang L, Wang Q, Song H, Zi G (2007) Organometallics 26:5323
Zi G, **ang L, Song H (2008) Organometallics 27:1242
Wang Q, **ang L, Song H, Zi G (2008) Inorg Chem 47:4319
Vitanova DV, Hampel F, Hultzsch KC (2011) J Organomet Chem 696:321
Benndorf P, Jenter J, Zielke L, Roesky PW (2011) Chem Commun 47:2574
Zhang Y, Yao W, Li H, Mu Y (2012) Organometallics 31:4670
Shibasaki M, Yoshikawa N (2002) Chem Rev 102:2187
Yamagiwa N, Matsunaga S, Shibasaki M (2004) Angew Chem Int Ed 43:4493
Hevia E, Mulvey RE (2011) Angew Chem Int Ed 50:6448
Seayad J, Tillack A, Hartung CG, Beller M (2002) Adv Synth Catal 344:795
Horrillo Martínez P, Hultzsch KC, Hampel F (2006) Chem Commun 2221
Ogata T, Ujihara A, Tsuchida S, Shimizu T, Kaneshige A, Tomioka K (2007) Tetrahedron Lett 48:6648
Deschamp J, Collin J, Hannedouche J, Schulz E (2011) Eur J Org Chem 3329
Horrillo-Martínez P (2008) Ph.D thesis, Universität Erlangen-Nürnberg
Harder S (2010) Chem Rev 110:3852
Barrett AGM, Crimmin MR, Hill MS, Procopiou PA (2010) Proc R Soc A 466:927
Crimmin MR, Hill MS (2013) Top Organomet Chem 45:191
Horrillo-Martínez P, Hultzsch KC (2009) Tetrahedron Lett 50:2054
Buch F, Harder S (2008) Z Naturforsch 63b:169
Neal SR, Ellern A, Sadow AD (2011) J Organomet Chem 696:228
Zhang X, Emge TJ, Hultzsch KC (2012) Angew Chem Int Ed 51:394
Nixon TD, Ward BD (2012) Chem Commun 48:11790
Wixey JS, Ward BD (2011) Chem Commun 47:5449
Wixey JS, Ward BD (2011) Dalton Trans 40:7693
Pohlki F, Doye S (2003) Chem Soc Rev 32:104
Bytschkov I, Doye S (2003) Eur J Org Chem 935
Doye S (2004) Synlett 1653
Odom AL (2005) Dalton Trans 225
Severin R, Doye S (2007) Chem Soc Rev 36:1407
Gribkov DV, Hultzsch KC (2004) Angew Chem Int Ed 44:5542
Bexrud JA, Beard JD, Leitch DC, Schafer LL (2005) Org Lett 7:1959
Kim H, Lee PH, Livinghouse T (2005) Chem Commun 5205
Roesky PW (2009) Angew Chem Int Ed 48:4892
Müller C, Saak W, Doye S (2008) Eur J Org Chem 2731
Bexrud JA, Eisenberger P, Leitch DC, Payne PR, Schafer LL (2009) J Am Chem Soc 131:2116
Knight PD, Munslow I, O'Shaughnessy PN, Scott P (2004) Chem Commun 894
Kissounko DA, Epshteyn A, Fettinger JC, Sita LR (2006) Organometallics 25:1076
Gribkov DV (2005) Ph.D thesis, Universität Erlangen-Nürnberg
Watson DA, Chiu M, Bergman RG (2006) Organometallics 25:4731
Zi G, Zhang F, Liu X, Ai L, Song H (2010) J Organomet Chem 695:730
**ang L, Zhang F, Zhang J, Song H, Zi G (2010) Inorg Chem Commun 13:666
Gott AL, Clarke AJ, Clarkson GJ, Scott P (2008) Chem Commun 1422
Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL (2007) Angew Chem Int Ed 46:354
Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL (2009) Angew Chem Int Ed 48:6938
Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL (2010) Angew Chem Int Ed 49:6475
Gott AL, Clarke AJ, Clarkson GJ, Scott P (2007) Organometallics 26:1729
Zi G, Zhang F, **ang L, Chen Y, Fang W, Song H (2010) Dalton Trans 39:4048
Reznichenko AL, Hultzsch KC (2010) Organometallics 29:24
Ayinla RO, Gibson T, Schafer LL (2011) J Organomet Chem 696:50
Walsh PJ, Baranger AM, Bergman RG (1992) J Am Chem Soc 114:1708
Baranger AM, Walsh PJ, Bergman RG (1993) J Am Chem Soc 115:2753
Walsh PJ, Hollander FJ, Bergman RG (1993) Organometallics 12:3705
Lee SY, Bergman RG (1995) Tetrahedron 51:4255
Polse JL, Andersen RA, Bergman RG (1998) J Am Chem Soc 120:13405
Pohlki F, Doye S (2001) Angew Chem Int Ed 40:2305
Straub BF, Bergman RG (2001) Angew Chem Int Ed 40:4632
Tobisch S (2007) Chem Eur J 13:4884
Allan LEN, Clarkson GJ, Fox DJ, Gott AL, Scott P (2010) J Am Chem Soc 132:15308
Stubbert BD, Marks TJ (2007) J Am Chem Soc 129:6149
Majumder S, Odom AL (2008) Organometallics 27:1174
Leitch DC, Payne PR, Dunbar CR, Schafer LL (2009) J Am Chem Soc 131:18246
Leitch D, Turner C, Schafer L (2010) Angew Chem Int Ed 49:6382
Leitch DC, Platel RH, Schafer LL (2011) J Am Chem Soc 133:15453
Manna K, Xu S, Sadow AD (2011) Angew Chem Int Ed 50:1865
Manna K, Everett WC, Schoendorff G, Ellern A, Windus TL, Sadow AD (2013) J Am Chem Soc 135:7235
Tobisch S (2012) Inorg Chem 51:3786
Anderson LL, Arnold J, Bergman RG (2004) Org Lett 6:2519
Anderson LL, Arnold J, Bergman RG (2006) Org Lett 8:2445
Anderson LL, Schmidt JAR, Arnold J, Bergman RG (2006) Organometallics 25:3394
Eisenberger P, Ayinla RO, Lauzon JMP, Schafer LL (2009) Angew Chem Int Ed 48:8361
Zi G, Zhang F, Song H (2010) Chem Commun 46:6296
Reznichenko AL, Emge TJ, Audörsch S, Klauber EG, Hultzsch KC, Schmidt B (2011) Organometallics 30:921
Zhang F, Song H, Zi G (2011) Dalton Trans 40:1547
Reznichenko AL, Hultzsch KC (2012) J Am Chem Soc 134:3300
Shen X, Buchwald SL (2010) Angew Chem Int Ed 49:564
Liu Z, Yamamichi H, Madrahimov ST, Hartwig JF (2011) J Am Chem Soc 133:2772
Kojima M, Mikami K (2012) Synlett 23:57
Turnpenny BW, Hyman KL, Chemler SR (2012) Organometallics 31:7819
Sherman ES, Fuller PH, Kasi D, Chemler SR (2007) J Org Chem 72:3896
Paderes MC, Belding L, Fanovic B, Dudding T, Keister JB, Chemler SR (2012) Chem Eur J 18:1711
Dalko PI, Moisan L (2004) Angew Chem Int Ed 43:5138
Seayad J, List B (2005) Org Biomol Chem 3:719
Akiyama T (2007) Chem Rev 107:5744
Schlummer B, Hartwig JF (2002) Org Lett 4:1471
Haskins CM, Knight DW (2002) Chem Commun 2724
Ackermann L, Kaspar LT, Althammer A (2007) Org Biomol Chem 5:1975
Ackermann L, Althammer A (2008) Synlett 995
McKinney Brooner RE, Widenhoefer RA (2011) Chem Eur J 17:6170
Anderson LL, Arnold J, Bergman RG (2005) J Am Chem Soc 127:14542
Marcseková K, Doye S (2007) Synthesis 145
Yang L, Xu L-W, **a C-G (2008) Tetrahedron Lett 49:2882
Shapiro ND, Rauniyar V, Hamilton GL, Wu J, Toste FD (2011) Nature 470:245
Brown AR, Uyeda C, Brotherton CA, Jacobsen EN (2013) J Am Chem Soc 135:6747
Hong S, Marks TJ (2002) J Am Chem Soc 124:7886
Hong S, Kawaoka AM, Marks TJ (2003) J Am Chem Soc 125:15878
Deschamp J, Olier C, Schulz E, Guillot R, Hannedouche J, Collin J (2010) Adv Synth Catal 352:2171
Lutete LM, Kadota I, Yamamoto Y (2004) J Am Chem Soc 126:1622
Kanno O, Kuriyama W, Wang ZJ, Toste FD (2011) Angew Chem Int Ed 50:9919
Dion I, Beauchemin AM (2011) Angew Chem Int Ed 50:8233
Butler KL, Tragni M, Widenhoefer RA (2012) Angew Chem Int Ed 51:5175
Cooke ML, Xu K, Breit B (2012) Angew Chem Int Ed 51:10876
Hoover JM, Petersen JR, Pikul JH, Johnson AR (2004) Organometallics 23:4614
Hickman AJ, Hughs LD, Jones CM, Li H, Redford JE, Sobelman SJ, Kouzelos JA, Johnson AR (2009) Tetrahedron Asymmetry 20:1279
Near KE, Chapin BM, McAnnally-Linz DC, Johnson AR (2011) J Organomet Chem 696:81
Hansen MC, Heusser CA, Narayan TC, Fong KE, Hara N, Kohn AW, Venning AR, Rheingold AL, Johnson AR (2011) Organometallics 30:4616
LaLonde RL, Sherry BD, Kang EJ, Toste FD (2007) J Am Chem Soc 129:2452
Zhang Z, Bender CF, Widenhoefer RA (2007) Org Lett 9:2887
Li H, Lee DL, Widenhoefer RA (2011) J Organomet Chem 696:316
Liu L-J, Wang F, Wang W, Zhao M-X, Shi M (2011) Beilstein J. Org Chem 7:555
LaLonde RL, Wang ZJ, Mba M, Lackner AD, Toste FD (2010) Angew Chem Int Ed 49:598
Hamilton GL, Kang EJ, Mba M, Toste FD (2007) Science 317:496
Rodríguez L-I, Roth T, Fillol JL, Wadepohl H, Gade LH (2012) Chem Eur J 18:3721
Teller H, Corbet M, Mantilli L, Gopakumar G, Goddard R, Thiel W, Fürstner A (2012) J Am Chem Soc 134:15331
Patil NT, Lutete LM, Wu H, Pahadi NK, Gridnev ID, Yamamoto Y (2006) J Org Chem 71:4270
Narsireddy M, Yamamoto Y (2008) J Org Chem 73:9698
Molander GA, Dowdy ED, Pack SK (2001) J Org Chem 66:4344
Arredondo VM, Tian S, McDonald FE, Marks TJ (1999) J Am Chem Soc 121:3633
Trost BM, Tang W (2003) J Am Chem Soc 125:8744
Trost BM, Tang W (2002) J Am Chem Soc 124:14542
Trost BM, Tang W, Toste FD (2005) J Am Chem Soc 127:14785
Ashby EC, Goel AB, DePriest RN (1981) J Org Chem 46:2429
Ashby EC, Goel AB, DePriest RN (1981) Tetrahedron Lett 22:4355
Newcomb M, Burchill MT, Deeb TM (1988) J Am Chem Soc 110:6528
Nishina N, Yamamoto Y (2006) Angew Chem Int Ed 45:3314
Sherry BD, Toste FD (2004) J Am Chem Soc 126:15978
Lathbury D, Gallagher T (1986) J Chem Soc Chem Commun 114
Ha JD, Cha JK (1999) J Am Chem Soc 121:10012
Pohlki F, Bytschkov I, Siebeneicher H, Heutling A, König WA, Doye S (2004) Eur J Org Chem 1967
Reznichenko AL, Hampel F, Hultzsch KC (2009) Chem Eur J 15:12819
Zhang Z, Bender CF, Widenhoefer RA (2007) J Am Chem Soc 129:14148
Ajamian A, Gleason JL (2004) Angew Chem Int Ed 43:3754
Fogg DE, dos Santos EN (2004) Coord Chem Rev 248:2365
Eilbracht P, Barfacker L, Buss C, Hollmann C, Kitsos-Rzychon BE, Kranemann CL, Rische T, Roggenbuck R, Schmidt A (1999) Chem Rev 99:3329
Eilbracht P, Schmidt AM (2006) Top Organomet Chem 18:65
Müller TE (2006) Top Organomet Chem 19:149
Heutling A, Pohlki F, Bytschkov I, Doye S (2005) Angew Chem Int Ed 44:2951
Mujahidin D, Doye S (2005) Eur J Org Chem 2689
Zhai H, Borzenko A, Lau YY, Ahn SH, Schafer LL (2012) Angew Chem Int Ed 51:12219
Liu X-Y, Che C-M (2009) Org Lett 11:4204
Han Z-Y, **ao H, Chen X-H, Gong L-Z (2009) J Am Chem Soc 131:9182
Noyori R (2002) Angew Chem Int Ed 41:2008
Kim H, Lim W, Im D, Kim D-G, Rhee YH (2012) Angew Chem Int Ed 51:12055
Acknowledgment
Generous financial support by the National Science Foundation through a NSF CAREER Award (CHE 0956021) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Reznichenko, A.L., Nawara-Hultzsch, A.J., Hultzsch, K.C. (2013). Asymmetric Hydroamination. In: Li, W., Zhang, X. (eds) Stereoselective Formation of Amines. Topics in Current Chemistry, vol 343. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2013_500
Download citation
DOI: https://doi.org/10.1007/128_2013_500
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-53928-2
Online ISBN: 978-3-642-53929-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)