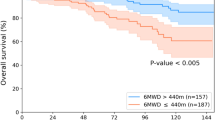
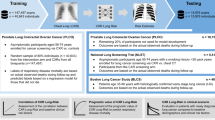
Abstract
Chronic obstructive pulmonary disease, generally known as COPD, is a progressive and eventually deadly lung condition that may be caused by emphysema and other illnesses. To detect these issues early and increase the patient's chance of survival, it is strongly advocated that chest X-rays be processed using very powerful image processing models. The geometry of the alveoli is harmed as a result of the bronchioles being narrowed and mucus-clogged in COPD. Utilizing sophisticated classification techniques for images, such as convolutional neural networks, one may see these shifts (CNN). When diagnosing COPD illnesses, CNNs have shown an accuracy of more than 95% when compared to static datasets. This accuracy is significantly impacted by variations in patient age, input image quality, capture angle, visual distortions, dataset type, and other elements. This paper provides a deep transfer learning-based incremental learning method for gradually updating the classification weights in the system. This method’s objective is to maintain the CNNs’ dependability over time. After analysing it on three different sets of data collected from X-rays of the lungs, the researchers found that the recommended model correctly recognised COPD in 99.5% of the instances. The results of this research suggest that when doing temporal analysis of COPD-detected images and calculating a patient’s projected life expectancy, Gated Recurrent Units (GRUs) should be employed. Medical practitioners utilise a process called lifetime analysis, which might result in more harsh treatment methods. As a result of patients being advised against continuous chest X-rays because to the long-term negative effects, a smaller dataset was used for the temporal analysis of COPD values, which produced an accuracy of 97% for lifetime analysis. Patients were advised against routine chest X-rays owing to their negative long-term effects.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rajasenbagam T et al (2021) Detection of pneumonia infection in lungs from chest X-Ray images using deep convolutional neural network and content-based image retrieval techniques. J Ambient Intell Humaniz Comput 2021:1–8. https://doi.org/10.1007/s12652-021-03075-2
Chagas JV et al (2021) A new approach for the detection of pneumonia in children using CXR images based on an real-time IoT system. J Real-Time Image Proc 18(4):1099–1114. https://doi.org/10.1007/s11554-021-01086-y
El Asnaoui K (2021) Design ensemble deep learning model for pneumonia disease classification. Int J Multimed Inform Retr 10(1):55–68. https://doi.org/10.1007/s13735-021-00204-7
López-Cabrera JD et al (2021) Current limitations to identify COVID-19 using artificial intelligence with chest X-ray imaging. Heal Technol 11(2):411–424. https://doi.org/10.1007/s12553-021-00520-2
Rahman S et al (2021) Deep learning-driven automated detection of COVID-19 from radiography images: a comparative analysis. Cogn Comput 2021:1–30. https://doi.org/10.1007/s12559-020-09779-5
Perumal V et al (2021) Detection of COVID-19 using CXR and CT images using transfer learning and haralick features. Appl Intell 51(1):341–358. https://doi.org/10.1007/s10489-020-01831-z
Qi X et al (2021) Chest X-ray image phase features for improved diagnosis of COVID-19 using convolutional neural network. Int J Comput Assist Radiol Surg 16(2):197–206. https://doi.org/10.1007/s11548-020-02305-w
Rashee J et al (2021) A machine learning-based framework for diagnosis of COVID-19 from chest X-ray images. Interdiscip Sci Comput Life Sci 13(1):103–117. https://doi.org/10.1007/s12539-020-00403-6
Ma J et al (2020) Survey on Deep Learning for Pulmonary Medical Imaging. Frontiers of Medicine 14(4):450–469. https://doi.org/10.1007/s11684-019-0726-4
Sultan LR et al (2021) Quantitative pleural line characterization outperforms traditional lung texture ultrasound features in detection of COVID-19. J Am College Emerg Phys Open 2(2):e12418. https://doi.org/10.1002/emp2.12418
Zheng S et al (2021) A dual-attention V-network for pulmonary lobe segmentation in CT scans. IET Image Proc 15(8):1644–1654. https://doi.org/10.1049/ipr2.12133
Irmak E (2021) COVID-19 disease severity assessment using CNN model. IET Image Proc 15(8):1814–1824. https://doi.org/10.1049/ipr2.12153
Sukanya Doddavarapu VN et al (2021) Rotational invariant fractional derivative filters for lung tissue classification. IET Image Proc 15(10):2202–2212. https://doi.org/10.1049/ipr2.12188
Klimeš F et al (2021) 3D Phase-resolved functional lung ventilation MR imaging in healthy volunteers and patients with chronic pulmonary disease. Magn Reson Med 85(2):912–925. https://doi.org/10.1002/mrm.28482
Roy S et al (2020) Deep learning for classification and localization of COVID-19 markers in point-of-care lung ultrasound. IEEE Trans Med Imaging 39(8):2676–2687. https://doi.org/10.1109/tmi.2020.2994459
Anthimopoulos M et al (2016) Lung pattern classification for interstitial lung diseases using a deep convolutional neural network. IEEE Trans Med Imag 35(5):1207–1216. https://doi.org/10.1109/tmi.2016.2535865
Joshua N, Stephen E et al (2021) 3D CNN with visual insights for early detection of lung cancer using gradient-weighted class activation. J Healthcare Eng 2021:6695518. https://doi.org/10.1155/2021/6695518
https://www.sciencedirect.com/science/article/pii/S2210670720308076. Accessed 15 Feb 2023
An F et al (2021) Medical image classification algorithm based on visual attention mechanism-MCNN. Oxid Med Cell Longev 2021:6280690. https://doi.org/10.1155/2021/6280690
Salamh S, Ahmed B et al (2021) A study of a new technique of the CT scan view and disease classification protocol based on level challenges in cases of coronavirus disease. Radiol Res Pract 2021:5554408. https://doi.org/10.1155/2021/5554408
Zak M, Krzyżak A (2020) Classification of lung diseases using deep learning models. In: Lecture notes in computer science. Springer International Publishing, pp 621–634. https://doi.org/10.1007/978-3-030-50420-5_47
Muthazhagan B et al (2020) An enhanced computer-assisted lung cancer detection method using content based image retrieval and data mining techniques. J Ambient Intell Humaniz Comput. https://doi.org/10.1007/s12652-020-02123-7
Trusculescu AA et al (2020) Deep learning in interstitial lung disease-how long until daily practice. Eur Radiol 30(11):6285–6292. https://doi.org/10.1007/s00330-020-06986-4
Farhat H et al (2020) Deep learning applications in pulmonary medical imaging: recent updates and insights on COVID-19. Mach Vis Appl 31(6):53. https://doi.org/10.1007/s00138-020-01101-5
Elaziz MA et al (2020) New machine learning method for image-based diagnosis of COVID-19. PLoS ONE 15(6):e0235187. https://doi.org/10.1371/journal.pone.0235187
Fan D-P et al (2020) Inf-Net: automatic COVID-19 lung infection segmentation from CT images. medRxiv. https://doi.org/10.1101/2020.04.22.20074948
Qin R et al (2020) Fine-grained lung cancer classification from PET and CT images based on multidimensional attention mechanism. Complexity 2020:1–12. https://doi.org/10.1155/2020/6153657
Gupta YK, Agrawal S (2021) A study of lung disease using image processing in big data environment. Mater Sci Eng 1022, 012030. https://doi.org/10.1088/1757-899x/1022/1/012030.
Du R et al (2020) Identification of COPD from multi-view snapshots of 3D lung airway tree via deep CNN. IEEE Access Pract Innov Open Solut 8:38907–38919. https://doi.org/10.1109/access.2020.2974617
Munawar F et al (2020) Segmentation of lungs in chest x-ray image using generative adversarial networks. IEEE Access Pract Innov Open Solut 8:153535–153545. https://doi.org/10.1109/access.2020.3017915
Li X et al (2021) A deep learning system that generates quantitative CT reports for diagnosing pulmonary tuberculosis. Appl Intell 51(6):4082–4093. https://doi.org/10.1007/s10489-020-02051-1
Mlodzinski E et al (2020) Machine learning for pulmonary and critical care medicine: a narrative review. Pulm Therap 6(1):67–77. https://doi.org/10.1007/s41030-020-00110-z
Shi H et al (2020) Multimodal lung tumor image recognition algorithm based on integrated convolutional neural network. Concurr Comput Pract Exp 32(21):e4965. https://doi.org/10.1002/cpe.4965
Peng J et al (2020) A machine-learning approach to forecast aggravation risk in patients with acute exacerbation of chronic obstructive pulmonary disease with clinical indicators. Sci Rep 10(1):3118. https://doi.org/10.1038/s41598-020-60042-1
Mei X et al (2020) Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat Med 26(8):1224–1228. https://doi.org/10.1038/s41591-020-0931-3
Hosseini MP et al (2012) Detection and severity scoring of chronic obstructive pulmonary disease using volumetric analysis of lung CT images. Iran J Radiol 9(1):22–27. https://doi.org/10.5812/iranjradiol.6759
Liu C et al (2020) A fully automatic segmentation algorithm for CT lung images based on random forest. Med Phys 47(2):518–529. https://doi.org/10.1002/mp.13939
Yamamoto S et al (2020) Pulmonary perfusion by chest digital dynamic radiography: comparison between breath-holding and deep-breathing acquisition. J Appl Clin Med Phys 21(11):247–255. https://doi.org/10.1002/acm2.13071
Kirby M et al (2020) Inter- and intra-software reproducibility of computed tomography lung density measurements. Med Phys 47(7):2962–2969. https://doi.org/10.1002/mp.14130
Hasse K et al (2020) Systematic feasibility analysis of performing elastography using reduced dose CT lung image pairs. Med Phys 47(8):3369–3375. https://doi.org/10.1002/mp.14112
Ahuja S et al (2021) Deep transfer learning-based automated detection of COVID-19 from lung CT scan slices. Appl Intell 51(1):571–585. https://doi.org/10.1007/s10489-020-01826-w
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Nair, S. (2023). DLLACC: Design of an Efficient Deep Learning Model for Identification of Lung Air Capacity in COPD Affected Patients. In: Choudrie, J., Mahalle, P.N., Perumal, T., Joshi, A. (eds) ICT for Intelligent Systems. ICTIS 2023. Smart Innovation, Systems and Technologies, vol 361. Springer, Singapore. https://doi.org/10.1007/978-981-99-3982-4_18
Download citation
DOI: https://doi.org/10.1007/978-981-99-3982-4_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4039-4
Online ISBN: 978-981-99-3982-4
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)