Abstract
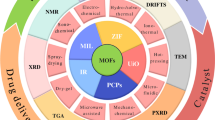
Metal–organic frameworks (MOFs), a family of porous crystalline materials generated by coordinated-metal ions in organic linkers, have gained more attention in the last decades due to their distinct structures and diverse uses. However, there are still technical obstacles to their practical implementation owing to MOFs’ inherent fragility and their tiny powder form, such as pipe obstruction, recovery challenges, and possible environmental toxicity. To get over these constraints, research has concentrated on ways to transform nanocrystalline MOFs into macroscopic materials. Recently, methods for forming MOFs into macrostructure beads (0D), nanofibers (1D), membranes (2D), gels/sponges (3D), and membranes with in situ growth or deposition of MOFs with polymers, cotton, foams, or other porous substrates have been devised. This chapter discusses the different uses of MOFs, including biochemistry, cancer treatment, the adsorption of pollutants, and others.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
current density (mA.cm−2), CN: cycle number. (*aqueous redox flow batteries).
- 2.
Vanadium redox flow battery.
- 3.
lithium metal batteries.
- 4.
sodium metal batteries.
- 5.
Secondary lithium-bromine (Li–Br2) batteries.
- 6.
lithium–sulfur batteries.
- 7.
Lithium-Ion Batteries.
- 8.
Ketjen Black (KB).
- 9.
Cellulose nanofiber.
- 10.
power conversion efficiency.
- 11.
short-circuit current density.
- 12.
open-circuit voltage.
- 13.
fill factor.
- 14.
Matrimid/NH2-PVDF (97/3) + 7 wt% NH2-MIL-101(Cr).
- 15.
polystyrene-acrylate (PSA) modified hollow ZIF-8 (PHZ).
- 16.
2D/2D FeNi-layered double hydroxide/bimetal-organic frameworks nanosheets.
- 17.
Tetracycline hydrochloride.
- 18.
Orange peel peroxidase.
- 19.
Methylene blue.
- 20.
Congo red.
- 21.
Tetracycline.
- 22.
Aerogels.
- 23.
P-nitrophenol.
- 24.
Sulfamethoxazole.
- 25.
Carbamazepine.
- 26.
Hydroxyapatite nanowires.
- 27.
Direct red 23.
- 28.
Diatom biosilica.
- 29.
Malachite green.
- 30.
Nanofibers.
- 31.
Ionic liquid.
References
Zhou, H.C., Long, J.R., Yaghi, O.M.: Introduction to metal-organic frameworks. Chem. Rev. 112(2), 673–674 (2012). https://doi.org/10.1021/cr300014x
Zhai, Q.G., Bu, X., Zhao, X., Li, D.S., Feng, P.: (2017) Pore space partition in metal-organic frameworks. Acc. Chem. Res. 50(2), 407–417 (2017). https://doi.org/10.1021/acs.accounts.6b00526
Deng, H., Doonan, C.J., Furukawa, H., Ferreira, R.B., Towne, J., Knobler, C.B., Wang, B., Yaghi, O.M.: Synthesis and characterization of metal-organic framework-74 containing 2, 4, 6, 8, and 10 different metals. Inorg. Chem. 53(12), 5881–5883 (2014). https://doi.org/10.1021/ic500434a
Shen, K., Chen, X., Chen, J., Li, Y.: Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 6(9), 5887–5903 (2016). https://doi.org/10.1021/acscatal.6b01222
Wang, H., Zhu, Q.L., Zou, R., Xu, Q.: Metal-organic frameworks for energy applications. Chem 2(1), 52–80 (2017). https://doi.org/10.1016/j.chempr.2016.12.002
Stock, N., Biswas, S.: Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 112, 933–969 (2012). https://doi.org/10.1021/cr200304e
Sun, Y.X., Sun, W.Y.: Influence of temperature on metal-organic frameworks. Chin. Chem. Lett. 25, 823–828 (2014). https://doi.org/10.1016/j.cclet.2014.04.032
Li, C., Shi, Y., Zhang, H., Zhao, Q., Xue, F., Li, X.: Cu-BTC metal-organic framework as a novel catalyst for low temperature selective catalytic reduction (SCR) of NO by NH3: promotional effect of activation temperature. Integr. Ferroelectr. 172, 169–179 (2016). https://doi.org/10.1080/10584587.2016.1177385
Canivet, J., Vandichel, M., Farrusseng, D.: Origin of highly active metal-organic framework catalysts: defects? Defects! Dalton Trans. 45, 4090–4099 (2016). https://doi.org/10.1039/C5DT03522H
Bang, J.H., Suslick, K.S.: Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 22, 1039 (2010). https://doi.org/10.1002/adma.200904093
Ahrenholtz, S.R., Landaverde-Alvarado, C., Whiting, M., Lin, S., Slebodnick, C., Marand, E., Morris, A.J.: Thermodynamic study of CO2 sorption by polymorphic microporous MOFs with open Zn (II) coordination sites. Inorg. Chem. 54, 4328–4336 (2015). https://doi.org/10.1021/ic503047y
Morris, R.E., Cejka, J.: Exploiting chemically selective weakness in solids as a route to new porous materials. Nat. Chem. 7, 381–388 (2015). https://doi.org/10.1038/nchem.2222
Diring, S., Furukawa, S., Takashima, Y., Tsuruoka, T., Kitagawa, S.: Controlled multiscale synthesis of porous coordination polymer in Nano/Micro regimes. Chem. Mater. 22, 4531 (2010). https://doi.org/10.1021/cm101778g
Wang, D., Huang, R., Liu, W., Sun, D., Li, Z.: Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 4, 4254–4260 (2014). https://doi.org/10.1021/cs501169t
**ao Zhang, B.S., Zhu, S., Xu, H., Tian, L.: UiO-66 and its Br-modified derivates for elemental mercury removal. J. Hazard. Mater. 320, 556–563 (2016). https://doi.org/10.1016/j.jhazmat.2016.08.039
Xu, C., Qiao, Z., Hou, B., Jiang, H., Gong, W., Dong, J., Li, H., Cui, Y., Liu, Y.: Chiral metal-organic frameworks with tunable catalytic selectivity in asymmetric transfer hydrogenation reactions. Nano Res. 14(2), 466-472 (2021). https://doi.org/10.1007/s12274-020-2905-7
Feng, X., Song, Y., Lin, W.: Dimensional reduction of Lewis acidic metal-organic frameworks for multicomponent reactions. J. Am. Chem. Soc. 143(21), 8184–8192 (2021). https://doi.org/10.1021/jacs.1c03561
Lei, Z., Deng, Y., Wang, C.: Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 6(7), 3258–3263 (2018). https://doi.org/10.1039/C7TA10566E
Akbarian, M., Sanchooli, E., Oveisi, A.L., Daliran, S.: chloride-coated UiO-66-Urea MOF: a novel multifunctional heterogeneous catalyst for efficient one-pot three-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 325, 115228 (2021). https://doi.org/10.1016/j.molliq.2020.115228
Ghobakhloo, F., Azarifar, D., Mohammadi, M., Keypour, H., Zeynali, H.: Copper (II) Schiff-base complex modified UiO-66-NH2 (Zr) metal–organic framework catalysts for Knoevenagel condensation–michael addition–cyclization reactions. Inorg. Chem. 61(12), 4825–4841 (2022). https://doi.org/10.1021/acs.inorgchem.1c03284
Jrada, A., Hmadeh, M., Awada, G., Chakleha, R., Ahmad, M.: Efficient biofuel production by MTV-UiO-66 based catalysts. Chem. Eng. J. 410, 128237 (2021). https://doi.org/10.1016/j.cej.2020.128237
Günsev, D., Sert, E.: Fuel additive synthesis by acetylation of glycerol using activated carbon/UiO-66 composite materials. Fuel 281, 118584 (2020). https://doi.org/10.1016/j.fuel.2020.118584
Li, X., Huang, L., Kochubei, A., Huang, J., Shen, W., Xu, H., Li, Q.: Evolution of a metal-organic framework into a Brønsted acid catalyst for glycerol dehydration to Acrolein. Chemsuschem 13(18), 5073–5079 (2020). https://doi.org/10.1002/cssc.202001377
Yılmaz, E., Sert, E., Atalay, S., Atalay, F.S.: Fabrication of chromium-based metal organic framework (MIL-101)/activated carbon composites for acetylation of glycerol. J. Taiwan Inst. Chem. Eng. 120, 93–105 (2021).https://doi.org/10.1016/j.jtice.2021.03.034
Gao, M.L., Qi, M.H., Liu, L., Han, Z.B.: An exceptionally stable core–shell MOF/COF bifunctional catalyst for a highly efficient cascade deacetalization–Knoevenagel condensation reaction. Chem. Commun. 55(45), 6377–6380 (2019). https://doi.org/10.1039/C9CC02174D
Guang, S.H., Tan, L.S., Zheng, Q., Paras, N.P.: Multiphoton absorbing materials: molecular designs, characterizations, and applications Chem. Rev. 108, 1245–1330 (2008). https://doi.org/10.1021/cr050054x
Zhan, W.W., Zhu, Q.L., Dang, S., Liu, Z., Kitta, M., Suenaga, K., Zheng, L.S., Xu, Q.: Synthesis of Highly active sub-nanometer Pt@Rh core–Shell Nanocatalyst via a photochemical route: Porous Titania Nanoplates as a superior photoactive support small nano micro 13, 1603879 (2017). https://doi.org/10.1002/smll.201603879
Bai, G., Tsang, M.K., Hao, J.: Tuning the luminescence of phosphors: beyond conventional chemical MethodAdv. Opt. Mater. 3, 431–462 (2015). https://doi.org/10.1002/adom.201400375
Cui, Y., Zhang, J., Hea, H., Qian: Photonic functional metal–organic frameworks. Chem. Soc. Rev. 47, 5740–5785 (2018).https://doi.org/10.1039/C7CS00879A
Cui, Y., Chen, B., Qian, G.: Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications Chem. Rev. 273–274, 76–86 (2014). https://doi.org/10.1016/j.ccr.2013.10.023
Rocha, J., Carlos, L.D., Paz, F.D., Ananias, D.: Luminescent multifunctional lanthanides-based metal–organic frameworks Chem. Soc. Rev. 40, 926–940 (2011). https://doi.org/10.1039/C0CS00130A
Rybak, J.C., Meyer, L.V., Wagenhofer, J., Sextl, G., Mu¨ller-Buschbaum, K.: Homoleptic Lanthanide 1,2,3-Triazolates ∞2–3[Ln (Tz*)3] and their diversified photoluminescence properties (2012) Inorg. Chem. 51, 13204–13213. https://doi.org/10.1021/ic301482e
Liu, W., Zhu, K., Teat, S.J., Dey, G., Shen, Z., Wang, L., O’Carroll, D.M., Li, J.: All-in-one: achieving robust, strongly luminescent and highly dispersible hybrid materials by combining ionic and coordinate bonds in molecular crystals. J. Am. Chem. Soc. 139, 9281–9290 (2017). https://doi.org/10.1021/jacs.7b04550
Weisheng Liu, T.J., Li, Y.I., Liu, Q., Tan, M., Wang, H., Wang, L.: Lanthanide coordination polymers and their Ag+-modulated fluorescence. J. Am. Chem. Soc. 126, 2280–2281 (2004). https://doi.org/10.1021/ja036635q
Hu, Z., Tan, K., Lustig, W.P., Wang, H., Zhao, Y., Zheng, C., Banerjee, D., Emge, T.J., Chabal, Y.J., Li, J.: Effective sensing of RDX via instant and selective detection of ketone vaporsChem. Science 5, 4873–4877 (2014).https://doi.org/10.1039/C4SC02157F
Hao, J.N., Yan, B.: Determination of Urinary 1-hydroxypyrene for biomonitoring of human exposure to polycyclic aromatic hydrocarbons carcinogens by a lanthanide-functionalized metal-organic framework sensor Adv. Funct. Mater. 27, 1603856 (2017). https://doi.org/10.1002/adfm.201603856
Sanda, S., Parshamoni, S., Biswas, S., Konar, S.: Highly selective detection of palladium and picric acid by a luminescent MOF: a dual functional fluorescent sensor. Chem. Commun. 51, 6576–6579 (2015). https://doi.org/10.1039/C4CC10442K
Buso, D., Jasieniak, J., Lay, M.D., Schiavuta, P., Scopece, P., Laird, J., Amenitsch, H., Hill, A.J., Falcaro, P.: Highly luminescent metal-organic frameworks through quantum dot do**. Small 2012(8), 80–88 (2012). https://doi.org/10.1002/smll.201100710
Lu, G., Li, S., Guo, Z., Farha, O.K., Hauser, B.G., Qi, X., Wang, Y., Wang, X., Han, S., Liu, X., DuChene, J.S., Zhang, H., Zhang, Q., Chen, X., Ma, J., Loo, S.C., Wei, W.D., Yang, Y., Hupp, J.T., Huo, F.: Facile synthesis of size-tunable ZIF-8 nanocrystals using reverse micelles as nanoreactorsNat. Chem. 4, 310–316 (2014). https://doi.org/10.1007/s11426-013-5008-4
He, H., Ma, E., Cui, Y., Yu, J., Yang, Y., Song, T., Wu, C.D., Chen, X., Chen, B., Qian, G.: Polarized three-photon-pumped laser in a single MOF microcrystal Nat. Commun. 7, 11087–11093 (2016). https://doi.org/10.1038/ncomms11087
Haoa, J.N., Yan, B.: Simultaneous determination of indoor ammonia pollution and its biological metabolite in the human body with a recyclable nanocrystalline lanthanide-functionalized MOF Nanoscale 8, 2881–2886 (2016). https://doi.org/10.1039/C5NR06066D
Zhang, C., Wang, B., Li, W., Huang, S., Kong, L., Li, Z., Li, L.: Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption Nat. Commun. 8, 1138–1146 (2017). https://doi.org/10.1038/s41467-017-01248-2
Li, B., Wen, H.M., Cui, Y., Zhou, W., Qian, G., Chen, B.: Emerging multifunctional metal-organic framework materials Adv. Mater. 28, 1–42 (2016). https://doi.org/10.1002/adma.201601133
Song, X.Z., Song, S.Y., Zhao, S.N., Hao, Z.M., Zhu, M., Meng, X., Wu, L.L., Zhang, H.J.: Single-crystal-to-single-crystal transformation of a europium (III) metal-organic framework producing a multi-responsive luminescent sensor Adv. Funct. Mater. 24, 4034–4041 (2014). https://doi.org/10.1002/adfm.201303986
Zhang, M., Feng, G., Song, Z., Zhou, Y.P., Chao, H.Y., Yuan, D., Tan, T.T., Guo, Z., Hu, Z., Tang, B.Z., Liu, B., Zhao, D.: Two-dimensional metal–organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc.136, 7241–7244 (2014).https://doi.org/10.1021/ja502643p
Douvali, A., Tsipis, A.C., Eliseeva, S.V., Petoud, S., Papaefstathiou, G.S., Malliakas, C.D., Papadas, I., Armatas, G.S., Margiolaki, I., Kanatzidis, M.G., Lazarides, T., Manos, M.J.: Turn-on luminescence sensing and real-time detection of traces of water in organic solvents by a flexible metal–organic framework. Angew. Chem. Int. Ed. 54, 1651–1656 (2015). https://doi.org/10.1002/ange.201410612
Falcaro, P., Ricco, R., Doherty, C.M., Liang, K., Hill, A.J., Styles, M.J.: MOF positioning technology and device fabrication. Chem. Soc. Rev. 43, 5513–5560 (20114). https://doi.org/10.1039/C4CS00089G
Yang, Q.Y., Pan, M., Wei, S.C., Li, K., Du, B.B., Su, C.Y.: Linear dependence of photoluminescence in mixed Ln-MOFs for color Tunability and barcode application Inorg. Chem. 54, 5707–5716 (2015). https://doi.org/10.1021/acs.inorgchem.5b00271
Zhou, Y., Yan, B.: Ratiometric multiplexed barcodes based on luminescent metal–organic framework films J. Mater. Chem. C 3, 8413–8418 (2015). https://doi.org/10.1039/C5TC01311A
Lustig, W.P., Wang, F., Teat, S.J., Hu, Z., Gong, Q., Li, J.: Chromophore-based luminescent metal-organic frameworks as lighting phosphors Inorg. Chem. 55, 7250–7256 (2016). https://doi.org/10.1021/acs.inorgchem.6b00897
Bachmann, V., Ronda, C., Meijerink, A.: Temperature quenching of yellow Ce3+ luminescence in YAG: Ce Chem. Mater. 21, 2077–2084 (2009). https://doi.org/10.1021/cm8030768
Lu, Z.Z., Zhang, R., Li, Y.Z., Guo, Z.J., Zheng, H.G.: Solvatochromic behavior of a nanotubular metal–organic framework for sensing small molecules. J. Am. Chem. Soc. 133, 4172–4174 (2011). https://doi.org/10.1021/ja109437d
Yang, C.X., Ren, H.B., Yan, X.P.: Fluorescent metal–organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 85, 7441–7446 (2013). https://doi.org/10.1021/ac401387z
Chen, L.F., Zheng, H.Z., Zhu, X., Lin, Z.Y., Guo, L.H., Qiu, B., Chen, G.N., Chen, Z.N.: Metal–organic frameworks-based biosensor for sequence-specific recognition of double-stranded DNA. Analyst 138, 3490–3493 (2013). https://doi.org/10.1039/C3AN00426K
Tan, H.L., Liu, B.X., Chen, Y.: Lanthanide coordination polymer nanoparticles for sensing of mercury (II) by photoinduced electron transfer. ACS Nano 6, 10505–10511 (2012). https://doi.org/10.1021/nn304469j
Shahat, A., Hassan, H.M.A., Azzazy, H.M.E.: Optical metal–organic framework sensor for selective discrimination of some toxic metal ions in water. Anal. Chim. Acta 793, 90–98 (2013). https://doi.org/10.1016/j.aca.2013.07.012
Liu, X.L., Zhao, X.J., Yang, X.X., Li, Y.F.: nanosized metal–organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 138, 4526–4531 (2013). https://doi.org/10.1039/C3AN00560G
Ma, W.J., Jiang, Q., Yu, P., Yang, L.F., Mao, L.Q.: Zeolitic imidazolate frameworkbased electrochemical biosensor for in vivo electrochemical measurements. Anal. Chem. 85, 7550–7557 (2013). https://doi.org/10.1021/ac401576u
Wang, Y., Wu, Y.C., **e, J., Ge, H.L., Hu, X.Y.: Multi-walled carbon nanotubes and metal–organic framework nanocomposites as novel hybrid electrode materials for the determination of nano-molar levels of lead in a lab-on-valve format. Analyst 138, 5113–5120 (2013). https://doi.org/10.1039/C3AN00598D
Hosseini, H., Ahmar, H., Dehghani, A., Bagheri, A., Tadjarod, A., Fakhari, A.R.: A novel electrochemical sensor based on metal–organic framework for electrocatalytic oxidation of L-cysteine. Biosens. Bioelectron. 42, 426–429 (2013). https://doi.org/10.1016/j.bios.2012.09.062
Hosseini, H., Ahmar, H., Dehghani, A., Bagheri, A., Fakhari, A.R., Amini, M.M.: Au- SH-SiO2 nanoparticles supported on metal-organic framework (Au-SHSiO2@Cu-MOF) as a sensor for electrocatalytic oxidation and determination of hydrazine. Electrochim. Acta 88, 301–309 (2013). https://doi.org/10.1016/j.electacta.2012.10.064
Wang, F., Zhao, J.B., Gong, J.M., Wen, L.L., Zhou, L., Li, D.F.: New multifunctional porous materials based on inorganic–organic hybrid single-walled carbon nanotubes: gas storage and high-sensitive detection of pesticides. Chem. Eur. J. 18, 11804–11810 (2012). https://doi.org/10.1002/chem.201200383
Lu, G., Hupp, J.T.: Metal–organic frameworks as sensors: a ZIF-8 based Fabry-Pérot device as a selective sensor for chemical vapors and gases. J. Am. Chem. Soc. 132, 7832–7833 (2010). https://doi.org/10.1021/ja101415b
Achmann, S., Hagen, G., Kita, J., Malkowsky, I.M., Kiener, C., Moos, R.: Metal-organic frameworks for sensing applications in the gas phase. Sensors,1574–1589 (2009). https://doi.org/10.3390/s90301574
Carrasco, J.M., Franquelo, L.G., Bialasiewicz, J.T., et al.: Power-electronic systems for the grid integration of renewable energy sources: A survey. IEEE Trans. Ind. Electron. 53, 1002–16 (2006). https://doi.org/10.1109/TIE.2006.878356
Zhang, Y., Lin, B., Wang, J., Tian, J., Sun, Y., Zhang, X., Yang, H.: All-solid-state asymmetric supercapacitors based on ZnO quantum dots/carbon/CNT and porous N-doped carbon/CNT electrodes derived from a single ZIF-8/CNT template. J. Mater. 1 Chem. A 42,10282–10293 (2016). https://doi.org/10.1039/C6TA03633C
Li, C., Liu, L., Kang, J., **ao, Y., Feng, Y., Cao, F.F., Zhang, H.: Pristine MOF and COF materials for advanced batteries. Energy Storage Mater. 31,115–134. https://doi.org/10.1016/j.ensm.2020.06.005
Guo, Y., Dai, Z., Lu, J., Zeng, X., Yuan, Y., Bi, X., Ma, L., Wu, T., Yan, Q., Amine, K.: Lithiation-induced non-noble metal nanoparticles for Li–O2 batteries ACS Appl. Mater. Inter. 11, 811–818 (2019). https://doi.org/10.1021/acsami.8b17417
Wu, D., Guo, Z., Yin, X., Pang, Q., Tu, B., Zhang, L., Wang, Y.G., Li, Q.: Metal-organic frameworks as cathode materials for Li–O2 batteries Adv. Mater. 26, 3258–3262 (2014). https://doi.org/10.1002/adma.201305492
Li, C., Liu, L., Kang, J., **ao, Y., Feng, Y., Cao, F.F., Zhang, H.: Pristine MOF and COF materials for advanced batteries. Energy Storage Mater. 31, 115–134 (2020), ISSN 2405–8297. https://doi.org/10.1016/j.ensm.2020.06.005
Hong, X.J., Song, C.L., Yang, Y., Tan, H.C., Li, G.H., Cai, Y.P., Wang, H.: Cerium based metal-organic frameworks as an efficient separator coating catalyzing the conversion of Polysulfides for high performance lithium-sulfur batteries. ACS Nano 13, 1923–1931 (2019). https://doi.org/10.1021/acsnano.8b08155
Bai, S., Zhu, K., Wu, S., Wang, Y., Yi, J., Ishida, M., Zhou, H.: A long-life lithium–sulphur battery by integrating zinc–organic framework-based separator. J. Mater. Chem. A 4, 16812–16817 (2016). https://doi.org/10.1039/C6TA07337A
Wang, Z., Huang, W., Hua, J., Wang, Y., Yi, H., Zhao, W., Zhao, Q., Jia, H., Fei, B., Pan, F.: An Anionic-MOF-based bifunctional separator for regulating lithium deposition and suppressing Polysulfides shuttle in Li–S batteries. Small Methods 4, 2000082 (2020). https://doi.org/10.1002/smtd.202000082
Dang, T., Zhang, G., Li, Q., Cao, Z., Zhang, G., Duan, H.: Ultrathin hetero-nanosheets assembled hollow Ni-Co-P/C for hybrid supercapacitors with enhanced rate capability and cyclic stability. J. Colloid Interface Sci. 577, 368–378 (2020). https://doi.org/10.1016/j.jcis.2020.05.065.
Zhang, F., Zhang, J., Ma, J., Zhao, Z., Li, Y., Li, R.: Polyvinylpyrrolidone (PVP) assisted in-situ construction of vertical metal-organic frameworks nanoplate arrays with enhanced electrochemical performance for hybrid supercapacitors. J. Colloid Interface Sci. 593, 32–40 (2021). https://doi.org/10.1016/j.jcis.2021.02.101
Zhang, Z., Huang, Y., Li, C., Li, X.: Metal-organic framework-supported poly (ethylene oxide) composite gel polymer electrolytes for high-performance lithium/sodium metal batteries. ACS Appl. Mater. Interfaces. 13(31), 37262–37272 (2021). https://doi.org/10.1021/acsami.1c11476
Peterson, B.B., Andrews, E.M., Hung, F., Flakea, J.C.: Carbonized metal-organic framework cathodes for secondary lithium-bromine batteries. J. Power Sources 492, 229658 (2021). https://doi.org/10.1016/j.jpowsour.2021.229658
Yang, D., Liang, Z., Tang, P., Zhang, C., Tang, M., Li, Q., Biendicho, J.J., Li, J., Heggen, M., Dunin-Borkowski, R.E., Xu, M., Llorca, J., Arbiol, J., Morante, J.R., Chou, S.L., Cabot, A.: A high conductivity one‐dimensional π‐d conjugated metal‐organic framework with efficient polysulfide trap**‐diffusion‐catalysis in lithium‐sulfur batteries. Adv. Mater., 2108835 (2022). https://doi.org/10.1002/adma.202108835
Li, X., He, C., Zheng, J., Wu, D., Duan, Y., Li, Y., Rao, P., Tang, B., Rui, Y.: Flocculent Cu caused by the Jahn-Teller effect improved the performance of Mg-MOF-74 as an anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces. 12(47), 52864–52872 (2020). https://doi.org/10.1021/acsami.0c17408
Capkováa, D., Almáši, M., Kazda, T., Čech, O., Király, N., Čudekc, P., Fedorkováa, A.S., Hornebecqd, V.: Metal-organic framework MIL-101 (Fe)–NH2 as an efficient host for sulphur storage in long-cycle Li–S batteries. Electrochim. Acta 354, 136640 (2020). https://doi.org/10.1016/j.electacta.2020.136640
**, L., Fu, Z., Qian, X., Li, F., Wang, Y., Wang, B., Shen, X.: Co-N/KB porous hybrid derived from ZIF 67/KB as a separator modification material for lithium-sulfur batteries. Electrochim. Acta 382, 138282 (2021). https://doi.org/10.1016/j.electacta.2021.138282
Qi, C., Xu, L., Wang, J., Li, H., Zhao, C., Wang, L., Liu, T.: Titanium-containing metal–organic framework modified separator for advanced lithium–sulfur batteries. ACS Sustain. Chem. Eng. 8(34), 12968–12975 (2020). https://doi.org/10.1021/acssuschemeng.0c03536
Zhang, F., Niu, T., Wu, F., Wu, L., Wang, G., Li, J.: Highly oriented MIL-101 (Cr) continuous films grown on carbon cloth as efficient polysulfide barrier for lithium-sulfur batteries. Electrochim. Acta 392, 139028 (2021). https://doi.org/10.1016/j.electacta.2021.139028
Sun, X., Xu, W., Zhang, X., Lei, T., Lee, S.Y., Wu, Q.: ZIF-67@ Cellulose nanofiber hybrid membrane with controlled porosity for use as Li-ion battery separator. J. Energy Chem. 52, 170–180 (2021). https://doi.org/10.1016/j.jechem.2020.04.057
Wang, L., Liu, H., Zhao, J., Zhang, X., Zhang, C., Zhang, G., Liu, Q., Duan, H.: Enhancement of charge transport in porous carbon nanofiber networks via ZIF-8-enabled welding for flexible supercapacitors. Chem. Eng. J. 382, 122979 (2020). https://doi.org/10.1016/j.cej.2019.122979
Zhang, D., Zhang, J., Pan, M., Wang, Y., Sun, T.: Necklace-like C-ZIF-8@ MWCNTs fabricated by electrochemical deposition towards enhanced supercapacitor. J. Alloy. Compd. 853, 157368 (2021). https://doi.org/10.1016/j.jallcom.2020.157368
Liu, H., Gong, L.G., Wang, C.X., Wang, C.M., Yu, Zhou, B.B.: {Cu 2 SiW 12 O 40} @ HKUST-1 synthesized by a one-step solution method with efficient bifunctional activity for supercapacitors and the oxygen evolution reaction. J. Mater. Chem. A 9(22), 13161–13169 (2021). https://doi.org/10.1039/D1TA01503F
Huang, S., Shi, X.R., Sun, C., Zhang, X., Huang, M., Liu, R., Wang, H., Xu, S.: Template-controlled in-situ growing of NiCo-MOF nanosheets on Ni foam with mixed linkers for high performance asymmetric supercapacitors. Appl. Surf. Sci. 572, 151344 (2022). https://doi.org/10.1016/j.apsusc.2021.151344
Kannangara, Y.Y., Rathnayake, U.A., Song, J.K.: Redox active multi-layered Zn-pPDA MOFs as high-performance supercapacitor electrode material. Electrochim. Acta 297, 145–154 (2019). https://doi.org/10.1016/j.electacta.2018.11.186
Shrivastav, V., Sundriyal, S.H., Kaur, A., Tiwari, U.K., Mishra, S., Deep, A.K.: Conductive and porous ZIF-67/PEDOT hybrid composite as superior electrode for all-solid-state symmetrical supercapacitors. J. Alloy. Compd. 843, 155992 (2020). https://doi.org/10.1016/j.jallcom.2020.155992
Ezeigwe, E.R., Dong, L., Wang, J., Wang, L., Yan, W., Zhang, J.: MOF-deviated zinc-nickel–cobalt ZIF-67 electrode material for high-performance symmetrical coin-shaped supercapacitors. J. Colloid Interface Sci. 574, 140–151 (2020). https://doi.org/10.1016/j.jcis.2020.04.025
Sundriyal, S., Shrivastav, V., Mishra, S., Deep, A.: Enhanced electrochemical performance of nickel intercalated ZIF-67/rGO composite electrode for solid-state supercapacitors. Int. J. Hydrogen Energy 45(55), 30859–30869 (2020). https://doi.org/10.1016/j.ijhydene.2020.08.075
Wang, G., Li, Y., Xu, L., **, Z., Wang, Y.: Facile synthesis of difunctional NiV LDH@ ZIF-67 pn junction: Serve as prominent photocatalyst for hydrogen evolution and supercapacitor electrode as well. Renew. Energy 162, 535–549 (2020). https://doi.org/10.1016/j.renene.2020.08.053
Yang, J., Chen, L., Li, W., Chen, G., Wang, L., Zhao, S.: A novel self-supported structure of Ce-UiO-66/TNF in a redox electrolyte with high supercapacitive performance. J. Colloid Interface Sci. 573, 55–61 (2020). https://doi.org/10.1016/j.jcis.2020.03.115
Wang, Y., Yue, Y., Yang, X., Han, L.: Toward long-term stable and highly efficient perovskite solar cells via effective charge transporting materials. Adv. Energy Mater. 8, 1800249 (2018). https://doi.org/10.1002/aenm.201800249
Wang, H., Zhu, C., Liu, L., Ma, S., Liu, P., Wu, J., Shi, C., Du, Q., Hao, Y., **ang, S., Chen, H., Chen, P., Bai, Y., Zhou, Y., Li, Q.: Patterns of use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers among patients with acute myocardial infarction in China from 2001 to 2011: China PEACE-Retrospective AMI study. Chen. Adv. Mater. 31, 1904408 (2019). https://doi.org/10.1161/JAHA.114.001343
Zhou, H.C., Long, J.R., Yaghi, O.M.: Introduction to metal–organic frameworks. Chem. Rev. 112(2), 673–674 (2012). https://doi.org/10.1021/cr300014x
Zhou, H.C., Kitagawa, S.: Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415 (2014). https://doi.org/10.1039/C4CS90059F
Ma, S., Zhou, H.C.: Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 46, 44 (2010). https://doi.org/10.1039/B916295J
Li, B., Wen, H.M., Cui, Y., Zhou, W., Qian, G., Chen, B.: Emerging multifunctional metal-organic framework materials Adv. Mater. 28, 8819 (2016). https://doi.org/10.1002/adma.201601133
Kreno, L.E., Leong, K., Farha, O.K., Allendorf, M., Van Duyne, R.P., Hupp, J.T.: Metal-organic framework materials as chemical sensors Chem. Rev. 112, 1105 (2011). https://doi.org/10.1021/cr200324t
Hu, Z., Deibert, B.J., Li, J.: Metal-organic framework materials as chemical sensors Chem. Soc. Rev. 43, 5815 (2014). https://doi.org/10.1021/cr200324t
Yamada, T., Otsubo, K., Makiura, R.: Kitagawa H (2013) Designer coordination polymers: dimensional crossover architectures and proton conduction Chem. Soc. Rev. 42, 6655 (2013). https://doi.org/10.1039/C3CS60028A
Horcajada, P., Gref, R., Baati, T., Allan, P.T., Maurin, G., Couvreur, P., Férey, G., Morris, R.E.: Metal-organic frameworks in Biomedicine Serre C Chem. Rev. 112, 1232 (2011). https://doi.org/10.1021/cr200256v
Fujita, M., Kwon, Y.J., Washizu, S., Ogura, K.: Preparation, Clathration ability, and catalysis of a two-dimensional square network material composed of Cadmium (II) and 4,4’-Bipyridine. J. Am. Chem. Soc. 116, 1151 (1994). https://doi.org/10.1021/ja00082a055
Farrusseng, D., Aguado, S., Pinel.: Metal–organic frameworks: opportunities for catalysis C. Angew. Chem. Int. Ed. 48, 7502 (2009). https://doi.org/10.1002/anie.200806063
Vielstich, W., Lamm, A., Gasteiger, H.A.: Handbook of Fuel Cells. Wiley, New York (2009)
Morozan, A., Jaouen, F.: Metal organic frameworks for electrochemical applications Energy Environ. Sci. 5, 9269 (2012). https://doi.org/10.1039/C2EE22989G
Agmon, N.: Structure and Energetics of the Hydronium Hydration Shells Chem. Phys. Lett. 244, 456 (1995). https://doi.org/10.1021/jp068960g
Rowsell, J.L.C., Yaghi, O.M.: Strategies for hydrogen storage in metal-organic frameworks Angew. Chem. Int. Ed. 44, 4670 (2005). https://doi.org/10.1002/anie.200462786
Lin, X., Telepeni, I., Blake, A.J., Dailly, A., Brown, C.M., Simmons, J.M., Zoppi, M., Walker, G.S., Thomas, K.M., Mays, T.J., Hubberstey, P., Champness, N.R., Schröder, M.: J. Am. Chem. Soc. 131, 2159 (2009). https://doi.org/10.1021/ja806624j
Kwak, W.J., Lau, K.C., Shin, C.D., Amine, K., Curtiss, L.A., Sun, Y.K.: A Mo2C/Carbon nanotube composite cathode for lithium–oxygen batteries with high energy efficiency and long cycle life. ACS Nano 9, 4129–4137 (2015). https://doi.org/10.1021/acsnano.5b00267
Mehtaba, T., Yasinb, G., Arifc, M., Shakeelc, M., Koraic, R.M., Nadeemd, M., Muhammadc, N., Lu, X.: Metal-organic frameworks for energy storage devices: batteries and supercapacitors. J. Energy Storage 21, 632–646 (2019). https://doi.org/10.1016/j.est.2018.12.025
Zhou, C., Longley, L., Krajnc, A., Smales, G.J., Qiao, A., Erucar, I., Doherty, C.M., Thornton, A.W., Hill, A.J., Ashling, C.W., Qazvini, O.T., Lee, S.J., Chater, P.A., Terrill, N.J., Smith, A.J., Yue, Y., Mali, G., Keen, D.A., Telfer, S.G., Bennett, T.D.: Nat. Commun. 9, 5042 (2018). https://doi.org/10.1038/s41467-018-07532-z
Liu, N., Cheng, J., Hu, L., Hou, W., Yang, X., Luo, M., Zhang, H., Ye, B., Zhou, J.: Boosting CO2 transport of poly (ethylene oxide) membranes by hollow Rubik-like “expressway” channels with anion pillared hybrid ultramicroporous materials. Chem. Eng. J. 427, 130845 (2022). https://doi.org/10.1016/j.cej.2021.130845
Rajati, H., Navarchian, A.H., Rodrigue, D., Tangestaninejad, S.H.: Improved CO2 transport properties of Matrimid membranes by adding amine-functionalized PVDF and MIL-101 (Cr). Sep. Purif. Technol. 235, 116149 (2020). https://doi.org/10.1016/j.seppur.2019.116149
Ding, R., Dai, Y., Zheng, W., Li, X., Yan, X., Liu, Y., Ruan, X., Li, S.H., Yang, X., Yang, K., He, G.: Vesicles-shaped MOF-based mixed matrix membranes with intensified interfacial affinity and CO2 transport freeway. Chem. Eng. J. 414, 128807 (2021). https://doi.org/10.1016/j.cej.2021.128807
Wang, B., Qiao, Z., Xu, J., Wang, J., Liu, X., Zhao, S., Wang, Z., Michael, D.: GuiverUnobstructed ultrathin gas transport channels in composite membranes by interfacial self-assembly. Adv. Mater. 32(22), 1907701 (2020). https://doi.org/10.1002/adma.201907701
Zhang, X., Lin, R.B., Wu, H., Huang, Y., Ye, Y., Duan, J., Zhou, W., Li, J.R., Chen, B.: Maximizing acetylene packing density for highly efficient C2H2/CO2 separation through immobilization of amine sites within a prototype MOF. Chem. Eng. J. 431, 134184 (2022). https://doi.org/10.1016/j.cej.2021.134184
Lin, Z.T., Liu, Q.Y., Yang, L., He, C.T., Li, L., Wang, Y.L.: Fluorinated Biphenyldicarboxylate-based metal-organic framework exhibiting efficient Propyne/Propylene separation. Inorg. Chem. 59(6), 4030–4036 (2020). https://doi.org/10.1021/acs.inorgchem.0c00003
Wang, G.D., Wang, H.H., Shi, W.J., Hou, L., Wang, Y.Y., Zhu, Z.: A highly stable MOF with F and N accessible sites for efficient capture and separation of acetylene from ternary mixtures. J. Mater. Chem. A 9(43), 24495–24502 (2021). https://doi.org/10.1039/D1TA05720K
Niu, Z., Cui, X., Pham, T., Verma, G., Lan, P.C., Shan, C., **ng, H., Forrest, K.A., Suepaul, S.H., Space, B., Nafady A,Al-Enizi, A.M., Ma, S.: A MOF‐based ultra‐strong acetylene Nano‐trap for highly efficient C2H2/CO2 Separation. Angewandte Chemie 133(10), 5343–5348 (2021). https://doi.org/10.1002/ange.202016225
Pei, J., Shao, K., Wang, J.X., Wen, H.M., Yang, Y., Cui, Y., Krishna, R., Li, B., Qian, G.: A Chemically stable hofmann-type metal− organic framework with sandwich-like binding sites for benchmark acetylene capture. Adv. Mater. 32(24), 1908275 (2020). https://doi.org/10.1002/adma.201908275
Zhang, Z., Peh, S.B., Krishna, R., Kang, C.H., Chai, K., Wang, Y., Shi, D., Zhao, D.: Optimal pore chemistry in an Ultramicroporous metal-organic framework for benchmark inverse CO2/C2H2 Separation. Angew. Chem. Int. Ed. 60(31), 17198–17204 (2021). https://doi.org/10.1002/anie.202106769
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37e8 (1972). https://doi.org/10.1038/238037a0
Guo, H., Lan, C., Zhou, Z., Sun, P., Wei, D., Li, C.: Molecular level distribution of black phosphorus quantum dots on nitrogen-doped graphene nanosheets for superior lithium storage. Small 12, 3748 (2016). https://doi.org/10.1016/j.nanoen.2016.10.019
Xu, H.Q., Hu, J., Wang, D., Zhaohui, L., Qun, Z., Yi, L., Yu, S.H., Jiang, H.J.: Visible- light photoreduction of CO2 in a metaleorganic framework: boosting electrone hole separation via electron trap states. Am. Chem. Soc. 137, 13440e3 (2015). https://doi.org/10.1021/jacs.5b08773
Wang, D.K., Huang, R., Liu, W., Sun, D., Li, Z.: Fe-Based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 4, 4254e60 (2014). https://doi.org/10.1021/cs501169t
Cardoso, J.C., Stulp, S., de Brito, J.F., Flor, J.B.S., Frem, R.C.G., Zanoni, M.V.B.: MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B 225, 563e73 (2018). https://doi.org/10.1016/j.apcatb.2017.12.013
Ojha, N., Kumar, S.: Tri-phase photocatalysis for CO2 reduction and N2 fixation with efficient electron transfer on a hydrophilic surface of transition-metal-doped MIL-88A (Fe). Appl. Catal. B 292, 120166 (2021). https://doi.org/10.1016/j.apcatb.2021.120166
Tang, Z., Zhu, F., Zhou, J., Chen, W., Wang, K.E., Liu, M., Wang, N., Li, N.: Monolithic NF@ ZnO/Au@ ZIF-8 photocatalyst with strong photo-thermal-magnetic coupling and selective-breathing effects for boosted conversion of CO2 to CH4. Appl. Catalysis B: Environ. 309, 121267 (2022). https://doi.org/10.1016/j.apcatb.2022.121267
Wang, G., He, C.T., Huang, R., Mao, J., Wang, D., Li, Y.: Photoinduction of Cu single atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels. J. Am. Chem. Soc. 142(45), 19339–19345 (2020). https://doi.org/10.1021/jacs.0c09599
Chen, X., Li, Q., Li, J., Chen, J., Jia, H.: Modulating charge separation via in situ hydrothermal assembly of low content Bi2S3 into UiO-66 for efficient photothermocatalytic CO2 reduction. Appl. Catal. B 270, 118915 (2020). https://doi.org/10.1016/j.apcatb.2020.118915
Ma, Y., Tang, Q., Sun, W.Y., Yao, Z.Y., Zhu, W., Li, T., Wang, J.: Assembling ultrafine TiO2 nanoparticles on UiO-66 octahedrons to promote selective photocatalytic conversion of CO2 to CH4 at a low concentration. Appl. Catal. B 270, 118856 (2020). https://doi.org/10.1016/j.apcatb.2020.118856
Wang, X., Wang, Y., Chai, G., Yang, G., Wang, C., Yan, W.: Poly (triphenylamine)-decorated UIO-66-NH2 mesoporous architectures with enhanced photocatalytic activity for CO2 reduction and H2 evolution. J. CO2 Utilization 51,101654 (2021). https://doi.org/10.1016/j.jcou.2021.101654
Wan, S., Ou, M., Zhong, Q., Wang, X.: Perovskite-type CsPbBr 3 quantum dots/UiO-66 (NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 358, 1287–1295 (2019). https://doi.org/10.1016/j.cej.2018.10.120
Dong, Y.J., Jiang, Y., Liao, J.F., Chen, H.Y., Kuang, D.B., Su, C.Y.: Construction of a ternary WO3/CsPbBr3/ZIF-67 heterostructure for enhanced photocatalytic carbon dioxide reduction. Science China Mater., 1–10 (2022). https://doi.org/10.1007/s40843-021-1962-9
Wang, L., Zhang, Z., Han, Q., Liu, Y., Zhong, J., Chen, J., Huang, J., She, H., Wang, Q.: Preparation of CdS-P25/ZIF-67 composite material and its photocatalytic CO2 reduction performance. Appl. Surf. Sci. 584, 152645 (2022). https://doi.org/10.1016/j.apsusc.2022.152645
Li, X., He, W., Li, C.H., Song, B.O., Liu, S.H.: Synergetic surface modulation of ZnO/Pt@ ZIF-8 hybrid nanorods for enhanced photocatalytic CO2 valorization. Appl. Catal. B 287, 119934 (2021). https://doi.org/10.1016/j.apcatb.2021.119934
Dhakshinamoorthy, A., Asiri, A.M., Garcia, H.: Tuneable nature of metal organic frameworks as heterogeneous solid catalysts for alcohol oxidation. Chem. Commun. 53, 10851–10869 (2017). https://doi.org/10.1039/C7CC05927B
Yang, D., Chen, Y., Su, Z., Zhang, X., Zhang, W., Srinivas, K.: Organic carboxylate-based MOFs and derivatives for electrocatalytic water oxidation. Coord. Chem. Rev. 428, 213619 (2021). https://doi.org/10.1016/j.ccr.2020.213619
Ali, M., Pervaiz, E., Noor, T., Rabi, O., Zahra, R., Yang, M.: Recent advancements in MOF-based catalysts for applications in electrochemical and photoelectrochemical water splitting: A review. Int. J. Energy Res. 45, 1190–1226 (2020). https://doi.org/10.1002/er.5807
Hunge, Y.M., Yadav, A.A., Mahadik, M.A., Bulakhe, R.N., Shim, J.J., Mathe, V.L., Bhosale, C.H.: Degradation of organic dyes using spray deposited nanocrystalline stratified WO3/TiO2 photoelectrodes under sunlight illumination. Opt. Mater. 76, 260–270 (2018). https://doi.org/10.1016/j.optmat.2017.12.044
Stavila, V., Talin, A.A., Allendorf, M.D.: MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 43(16), 5994–6010 (2014). https://doi.org/10.1039/c4cs00096j
Ma, L., Gao, J., Huang, C., Xu, X., Xu, L.U., Ding, R., Bao, H., Wang, Z., Xu, G., Li, Q., Deng, P., Ma, H.: UiO-66-NH-(AO) MOFs with a New Ligand BDC-NH-(CN) for efficient extraction of uranium from seawater. ACS Appl. Mater. Interfaces. 13(48), 57831–57840 (2021). https://doi.org/10.1021/acsami.1c18625
Yuan, G., Yu, Y., Li, J., Jiang, D., Gu, J., Tang, Y.I., Qiu, H., **ong, W., Liu, N.: Facile fabrication of a noval melamine derivative-doped UiO-66 composite for enhanced Co (II) removal from aqueous solution. J. Mol. Liq. 328, 115484 (2021). https://doi.org/10.1016/j.molliq.2021.115484
Huang, M., Lou, Z., Zhao, W., Lu, A., Hao, X., Wang, Y., Feng, X., Shan, W., **ong, Y.: Immersion grinding and in-situ polymerization synthesis of poly (ionic liquid) s incorporation into MOF composites as radioactive TcO4-scavenger. J. Hazard. Mater. 422, 126871 (2022). https://doi.org/10.1016/j.jhazmat.2021.126871
Ahmadijokani, F., Tajahmadi, S., Bahi, A., Molavi, H., Rezakazemi, M., Ko, F., Aminabhavi, T.M., Arjmand, M.: Ethylenediamine-functionalized Zr-based MOF for efficient removal of heavy metal ions from water. Chemosphere 264, 128466 (2021). https://doi.org/10.1016/j.chemosphere.2020.128466
Zhong, X., Liu, Y., Liang, W., Zhu, Y., Hu, B.: Construction of core-shell MOFs@ COF hybrids as a platform for the removal of UO22+ and Eu3+ Ions from Solution. ACS Appl. Mater. Interfaces. 13(11), 13883–13895 (2021). https://doi.org/10.1021/acsami.1c03151
Jia, W., Hu, X.: Metal selectivity and effects of co-existing ions on the removal of Cd, Cu, Ni, and Cr by ZIF-8-EGCG nanoparticles. J. Colloid Interface Sci. 589, 578–586 (2021). https://doi.org/10.1016/j.jcis.2021.01.021
Chen, C., Liu, Q., Chen, W., Li, F., **ao, G., Chen, C., Li, R., Zhou, J.: A high absorbent PVDF composite membrane based on β-cyclodextrin and ZIF-8 for rapid removing of heavy metal ions. Separation and Purification Technology: 120993 (2022). https://doi.org/10.1016/j.seppur.2022.120993
Ahmad, K., Shah, H., Ashfaq, M., Shah, S.S.A., Hussain, E., Naseem, H.A., Parveen, S., Ayub, A.: Effect of metal atom in zeolitic imidazolate frameworks (ZIF-8 & 67) for removal of Pb2+ & Hg2+ from water. Food Chem. Toxicol. 149, 112008 (2021). https://doi.org/10.1016/j.fct.2021.112008
Poudel, B.M., Awasthi, G.P., Kim, H.O.: Novel insight into the adsorption of Cr (VI) and Pb (II) ions by MOF derived Co-Al layered double hydroxide@ hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 417, 129312 (2021). https://doi.org/10.1016/j.cej.2021.129312
Omer, A.M., Abd El-Monaem, E.M., Abd El-Latif, M.M., El-Subruiti, G.M., Eltaweil, A.S.: Facile fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr (VI) from aqueous solutions. Carbohyd. Polym. 265, 118084 (2021). https://doi.org/10.1016/j.carbpol.2021.118084
Wang, Y., Peh, S.B., Zhao, D.: Alternatives to cryogenic distillation: advanced porous materials in adsorptive light olefin/paraffin separations. Small 15, 1900058 (2019). https://doi.org/10.1002/smll.201900058
Bari, M.A., Kindzierski, W.B.: (2018) Ambient volatile organic compounds (VOCs) in communities of the Athabasca oil sands region: Sources and screening health risk assessment Environ. Pollut. 235, 602–614 (2018). https://doi.org/10.1016/j.envpol.2017.12.065
Molla, M.A.I., Tateishi, I., Furukawa, M., Katsumata, H., Suzuki, T., Kaneco, S.: Photocatalytic Decolorization of dye with self-dye-sensitization under fluorescent light irradiation open. J. Inorg. Non- Met. Mater. 7, 1–7 (2017). https://doi.org/10.3390/chemengineering1020008
Wu, Y., Li, X., Zhao, H., Yao, F., Cao, J., Chen, Z., Ma, F., Wang, D., Yang, Q.: 2D/2D FeNi-layered double hydroxide/bimetal-MOFs nanosheets for enhanced photo-Fenton degradation of antibiotics: Performance and synergetic degradation mechanism. Chemosphere 287, 132061 (2022). https://doi.org/10.1016/j.chemosphere.2021.132061
Salgaonkar, M., Nadar, S.S., Rathod, V.K.: Biomineralization of orange peel peroxidase within metal organic frameworks (OPP–MOFs) for dye degradation. J. Environ. Chem. Eng. 7(2), 102969 (2019). https://doi.org/10.1016/j.jece.2019.102969
Hu, T., Deng, F., Feng, H., Zhang, J., Shao, B., Feng, C., Tang, W., Tang, L.: Fe/Co bimetallic nanoparticles embedded in MOF-derived nitrogen-doped porous carbon rods as efficient heterogeneous electro-Fenton catalysts for degradation of organic pollutants. Appl. Mater. Today 24, 101161 (2021). https://doi.org/10.1016/j.apmt.2021.101161
Ren, W., Gao, J., Lei, C., **e, Y., Cai, Y., Ni, Q., Yao, J.: Recyclable metal-organic framework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants. Chem. Eng. J. 349, 766–774 (2018). https://doi.org/10.1016/j.cej.2018.05.143
Abazari, R., Morsali, A., Dubal, D.P.: An advanced composite with ultrafast photocatalytic performance for the degradation of antibiotics by natural sunlight without oxidizing the source over TMU-5@ Ni–Ti LDH: mechanistic insight and toxicity assessment. Inorganic Chem. Front. 7(12), 2287–2304 (2020). https://doi.org/10.1039/D0QI00050G
Samy, M., Ibrahim, M.G., Fujii, M., Diab, K.E., ElKady, M., Alalm, M.G.: CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 406, 127152 (2021). https://doi.org/10.1016/j.cej.2020.127152
Li, B., Li, Y., Ren, J., Dai, F., Zhang, Y., He, Y., Song, P., Wang, R.: Hydroxyapatite coated with co-based metal organic framework nanoparticles as heterojunctions for catalytic degradation of organics. ACS Appl. Nano Mater. 4(9), 9370–9381 (2021). https://doi.org/10.1021/acsanm.1c01839
Bagherzadeh, S.B., Kazem Eini, M., Mahmoodi, N.M.: A study of the DR23 dye photocatalytic degradation utilizing a magnetic hybrid nanocomposite of MIL-53 (Fe)/CoFe2O4: facile synthesis and kinetic investigations. J. Mol. Liq. 301, 112427 (2020). https://doi.org/10.1016/j.molliq.2019.112427
Uthappa, U.T., Sriram, G., Arvind, O.R., Kumar, S., Jung, H.Y., Neelgund, G.M., Losic, D., Kurkuri, M.D.: Engineering MIL-100 (Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl. Surf. Sci. 528, 146974 (2020). https://doi.org/10.1016/j.apsusc.2020.146974
Chandra, R., Mala.: Controlled synthesis of AgNPs@ ZIF-8 composite: efficient heterogeneous photocatalyst for degradation of methylene blue and congo red. J. Water Process. Eng. 36, 101266 (2020). https://doi.org/10.1016/j.jwpe.2020.101266
Zhan, Y., Lan, J., Shang, J., Yang, L., Guan, X., Li, W., Chen, S., Qi, Y., Lin, S.: Durable ZIF-8/Ag/AgCl/TiO2 decorated PAN nanofibers with high visible light photocatalytic and antibacterial activities for degradation of dyes. J. Alloy. Compd. 822, 153579 (2020). https://doi.org/10.1016/j.jallcom.2019.153579
Müller, K., Fink, K., Schöttner, L., Koenig, M., Heinke, L., Wöll, C.: Defects as color centers: the apparent color of metal-organic frameworks containing Cu2+-based paddle-wheel units ACS Appl. Mater. Interfaces 9, 37463 (2017). https://doi.org/10.1021/acsami.7b12045
Qian, Q., Asinger, P.A., Lee, M.J., Han, G., Rodriguez, K.M., Lin, S., Benedetti, F.M., Wu, A.X., Chi, W.S., Smith, Z.P.: MOF-based membranes for gas separations. Chem. Rev. 120(16), 8161–8266 (2020). https://doi.org/10.1021/acs.chemrev.0c00119
McKinlay, A.C., Morris, R.E., Horcajada, P., Férey, G., Gref, R., Couvreur, P., Serre, C.: BioMOFs: Metal−organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 49, 6260–6266 (2010). https://doi.org/10.1002/anie.201000048
Yang, J., Yang, Y.W.: Metal−organic frameworks for biomedical applications. Small 16, 1906846 (2020). https://doi.org/10.1002/smll.201906846
Kitagawa, S., Kitaura, R., Noro, S.I.: Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 43, 2334−2375 (2004). https://doi.org/10.1002/anie.200300610
Zhu, X., Li, S., Shi, Y., Cai, N.: Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog. Energy Combust. Sci. 75, 100784 (2019). https://doi.org/10.1016/j.pecs.2019.100784
Barelli, L., Bidini, G., Gallorini, F., Servili, S.: Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: a review. Energy 33(4), 554–570 (2008). https://doi.org/10.1016/j.energy.2007.10.018
Sanders, D.F., Smith, Z.P., Guo, R., Robeson, L.M., McGrath, J.E., Paul, D.R., Freeman, B.D.: Energy-efficient polymeric gas separation membranes for a sustainable future: a review. Polymer 54(18), 4729–4761 (2013). https://doi.org/10.1016/j.polymer.2013.05.075
Worrell, E., Phylipsen, D., Einstein, D., Martin, N.: Energy use and energy intensity of the US chemical industry (No. LBNL-44314). Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States) (2000). https://doi.org/10.2172/773773
Sholl, D.S., Lively, R.P.: Seven chemical separations to change the world. Nature 532(7600), 435–437 (2016)
Worrell, E., Carreon, J.R.: Energy demand for materials in an international context. Philos. Trans. Royal Soc. A Math., hil. Trans. R Soc. A 375(2095), 20160377 (2017). https://doi.org/10.1098/rsta.2016.0377
Keskin, S., Sholl, D.S.: Screening metal−organic framework materials for membrane-based methane/carbon dioxide separations. J. Phys. Chem. C 111(38), 14055–14059 (2007). https://doi.org/10.1021/jp075290l
Arnold, M., Kortunov, P., Jones, D.J., Nedellec, Y., Kärger, J., Caro, J.: Oriented crystallisation on supports and anisotropic mass transport of the metal-organic framework manganese formate. Eur. J. Inorg. Chem 2007, 60 (2007). https://doi.org/10.1002/ejic.200600698
Bux, H., Liang, F., Li, Y., Cravillon, J., Wiebcke, M., Caro, J.: Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 131(44), 16000–16001 (2009). https://doi.org/10.1021/ja907359t
Marti, A.M., Venna, S.R., Roth, E.A., Culp, J.T., Hopkinson, D.P.: Simple fabrication method for mixed matrix membranes with in situ MOF growth for gas separation. ACS Appl. Mater Interfaces 10(29), 24784–24790 (2018). https://doi.org/10.1021/acsami.8b06592
Bennett, T.D., Tan, J.C., Yue, Y., Baxter, E., Ducati, C., Terrill, N.J., … Greaves, G.N.: Hybrid glasses from strong and fragile metal-organic framework liquids. Nat. Commun. 6(1), 1–7 (2015). https://doi.org/10.1038/ncomms9079
Bennett, T.D., Yue, Y., Li, P., Qiao, A., Tao, H., Greaves, N.G., ... Keen, D.A.: Melt-quenched glasses of metal–organic frameworks. J. Am. Chem. Soc. 138(10), 3484–3492 (2016). https://doi.org/10.1021/jacs.5b13220
Ka, G.R.P.P.B., Chapman, K.W., Keen, D.A., Bennett, T.D., Coudert, F.-X.: Nat. Mater 16, 1149–1154 (2017)
Pisklak, T.J., Macías, M., Coutinho, D.H., Huang, R.S., Balkus, K.J.: Hybrid materials for immobilization of MP-11 catalyst. Top. Catal. 38(4), 269–278 (2006). https://doi.org/10.1007/s11244-006-0025-6
Chen, Y., Lykourinou, V., Vetromile, C., Hoang, T., Ming, L.J., Larsen, R.W., Ma, S.: How can proteins enter the interior of a MOF? Investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J. Am. Chem. Soc. 134(32), 13188–13191 (2012). https://doi.org/10.1021/ja305144x
Hou, C., Wang, Y., Ding, Q., Jiang, L., Li, M., Zhu, W., ... Liu, M.: Facile synthesis of enzyme-embedded magnetic metal–organic frameworks as a reusable mimic multi-enzyme system: mimetic peroxidase properties and colorimetric sensor. Nanoscale 7(44), 18770–18779 (2015). https://doi.org/10.1039/C5NR04994F
Wang, S., McGuirk, C.M., Ross, M.B., Wang, S., Chen, P., **ng, H., ... Mirkin, C.A.: General and direct method for preparing oligonucleotide-functionalized metal–organic framework nanoparticles. J. Am. Chem. Soc. 139(29), 9827–9830 (2017). https://doi.org/10.1021/jacs.7b05633
An, J., Geib, S.J., Rosi, N.L.: Cation-triggered drug release from a porous zinc−adeninate metal−organic framework. J. Am. Chem. Soc. 131, 8376–8377 (2009). https://doi.org/10.1021/ja902972w
Rejman, J., Oberle, V., Zuhorn, I.S., Hoekstra, D.: Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 377(1), 159–169 (2004). https://doi.org/10.1042/bj20031253
McMahon, H T., Boucrot, E.: Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol1. 2(8), 517–533 (2011). https://doi.org/10.1038/nrm3151
Horcajada, P., Gref, R., Baati, T., Allan, P.K., Maurin, G., Couvreur, P., ... Serre, C.: Metal–organic frameworks in biomedicine. Chem. Rev. 112(2), 1232–1268 (2012). https://doi.org/10.1021/cr200256v
Han, W., Wu, Z., Li, Wang, Y.: Graphene family nanomaterials (GFNs)—promising materials for antimicrobial coating and film: a review. Chem. Eng. J. 358, 1022–1037 (2019).https://doi.org/10.1016/j.cej.2018.10.106
Dong, Y., Wu, X., Chen, X., Zhou, P., Xu, F., Liang, W.: Nanotechnology sha** stem cell therapy: recent advances, application, challenges, and future outlook. Biomed. Pharmacother. 137, 111236 (2021). https://doi.org/10.1016/j.biopha.2021.111236
Winkler, T., Sass, F.A., Duda, G.N., Schmidt-Bleek, K.: A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: the unsolved challenge. Bone J. Res. 7(3), 232 e 243 (2018). https://doi.org/10.1302/2046-3758.73.BJR-2017-0270.R1
Dan, W., Dai, J.: Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem. 187, 111980 (2020). https://doi.org/10.1016/j.ejmech.2019.111980
Baht, G.S., Vi, L., Alman, B.A.: The role of the immune cells in fracture healing. Curr. Osteoporos Rep. 16(2), 138 e 145 (2018). https://doi.org/10.1007/s11914-018-0423-2
**a, Y., Fan, X., Yang, H., Li, L., He, C., Cheng, C., Haag, R.: ZnO/Nanocarbons-modified fibrous scaffolds for stem cell-based osteogenic differentiation. Small 16(38), 2003010 (2020). https://doi.org/10.1002/smll.202003010
Sivaranjani, V., Philominathan, P.J.W.M.: Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med 12, 1–5 (2016). https://doi.org/10.1016/j.wndm.2015.11.002
Ash, C., Stone, R.J.S.: A question of dose: introduction. (Metals: impacts on health and the environment: special section). Science 300(5621), 925e926 (2003)
Veith, A.P., Henderson, K., Spencer, A., Sligar, A.D., Baker, A.B.: Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 146, 97–125 (2019). https://doi.org/10.1016/j.addr.2018.09.010
**ao, J., Zhu, Y., Huddleston, S., Li, P., **ao, B., Farha, O.K., Ameer, G.A.: Copper metaleorganic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 12(2), 1023e1032 (2018). https://doi.org/10.1021/acsnano.7b01850
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salehi, M.M., Esmailzadeh, F., Hassanzadeh-Afruzi, F. (2023). Applications of MOFs. In: Maleki, A., Taheri-Ledari, R. (eds) Physicochemical Aspects of Metal-Organic Frameworks. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-18675-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-18675-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18674-5
Online ISBN: 978-3-031-18675-2
eBook Packages: EngineeringEngineering (R0)