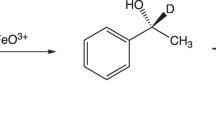
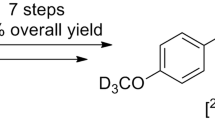
Abstract
In modern pharmaceutical research practice an everincreasing emphasis is being placed on studies of drug dynamics, that is, the absorption, distribution, metabolism, and elimination of an administered drug. Radiocarbon labeling can be one of the most valuable tools for such studies, provided that the drug to be studied can be synthesized containing the radiocarbon label. Fortunately, for one very large class of organic compounds of physiological interest, the N-methyl-amines, a wide variety of labeling procedures are available. Indeed, among the earliest of radiocarbon-labeled drugs to be prepared were meperidine-N-methyl-C14 (1), dimethylaminoazobenzene-N-methyl-C14 (2), and morphine-N-methyl-C14 (3).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Tarpey, W., Hauptmann, H., Tolbert, B.M., and Rapoport, H., J. Am. Chem. Soc. 72: 5126 (1950).
Boissonas, R. A., Turner, R. A., and du Vigneaud, V., J. Biol. Chem. 180: 1053 (1949).
Rapoport, H., Lovell, C.H., and Tolbert, B.M., J. Am. Chem. Soc. 73: 5900 (1951).
Hansson, E., and Schmiterlöw, C.G., J. Pharmacol. 137: 91 (1962).
Ross, J.L., Young, R. L., and Maass, A.R., Science 128: 1279 (1958).
Ott, D.G., in: Organic Synthesis with Isotopes, Interscience, New York, 1958.
Andersen, K.S., and Woods, L.A., J. Org. Chem. 24: 274 (1959).
Flynn, E.H., Murphy, H.W., and McMahon, R.E., J. Am. Chem. Soc. 77: 3104 (1955).
Pohland, A., Sullivan, H.R., and McMahon, R.E., J. Am. Chem. Soc. 79: 1442 (1957).
McMahon, R.E., Culp, H.W., and Marshall, F. J., J. Pharmacol. 149: 436 (1965).
McMahon, R.E., J. Med. Pharm. Chem. 4: 67 (1961).
Woods, L.A., Mellen, L.B., and Andersen, K.S., J. Pharmacol. 124: 1 (1958).
Kristerson, L., Hoffman, P., and Hansson, E., Acta Pharmacol. Toxicol. 22: 205 (1965).
Foreman, W.W., Murray III, A., and Ronzio, A.R., J. Org. Chem. 15: 119 (1950).
Fodor, E., Janzso, G., Otvos, L., and Benfi, D., Ber. 93: 268 (1960).
Zehnder, K., Kalberer, F., Kreis, W., and Rutschmann, J., Biochem. Pharmacol. 11: 535 (1962).
Aebi, H., Sauber, E., Lehner, H., and Michaelis, W., Arzreimittel Forsch. 14: 92 (1964).
Leete, E., and Bell, V.M., J. Am. Chem. Soc. 81: 4358 (1959).
Miller, E.C., Plescia, A.M., Miller, J.A., and Heidelberger, C., J. Biol. Chem. 196: 863 (1962).
Berenbom, M., Can. Res. 22: 1343 (1962).
McMahon, R.E., and Easton, N.R., J. Pharmacol. 135: 128 (1962).
Werner, G., and Mohammad, N., Ann. 964: 157 (1966).
Werner, G., Schmidt, H.L., and Kossner, E., Ann. 644: 109 (1961).
El-Merzabani, M.M., Chem. Pharm. Bull. (Japan) 13: 1362 (1965).
Artom, C., and Crowder, M., J. Am. Chem. Soc. 74: 2412 (1952).
Easton, N.R., Lukach, C.A., Nelson, S.J., and Fish, V.B., J. Am. Chem. Soc. 80: 2519 (1958).
McMahon, R.E., Marshall, F.J., Culp, H.W., and Miller, W.M., Biochem. Pharmacol. 12: 1207 (1963).
Elison, C., Elliott, H.W., Look, M., and Rapoport, H., J. Med. Chem. 6: 237 (1963).
Cf. Chem. Engr. News, February 8, 1960, p. 51.
Marshall, F.J., McMahon, R.E., and Jones, R.G., J. Agr. Food Chem. 14: 498 (1966).
Little, J.N., and Ray, F.E., J. Am. Chem. Soc. 74: 4955 (1952).
Searle, N.E., and Cupery, H.E., J. Org. Chem. 19: 1622 (1954).
Marer, W., Neumann, D., Schröter, H.B., and Schutte, H.R., Z. Chem. 6: 341 (1966).
Yoneda, F., Suzuki, K., and Nitta, Y., J. Am. Chem. Soc. 88: 2328 (1966).
Pohland, A., Sullivan, H.R., and Lee, H.M., Absts. 136th Mtg. Am. Chem. Soc. p. 15 - 0, September 1959.
Fornefeld, E. J., Sullivan, H.R., and Thompson, W.E., U.S. Patent 3, 213, 128.
Cope, A.C., Cigonek, E., Fleckenstein, L.N., and Meisinger, M.A.P., J. Am. Chem. Soc. 82: 4651 (1960).
Pichat, L., Audinot, M., and Monnet, J., Bull. Soc. Chim. 85 (1954).
Buck, J.S., J. Am. Chem. Soc. 52: 419 (1930).
Sekiya, M., and Ito, K., Chem. Pharm. Bull. (Japan) 14: 1007 (1966).
Axelrod, J., Pharmacol. Rev. 18: 95 (1966).
Axelrod, J., J. Biol. Chem. 237: 1657 (1962).
Fuller, R.W., and Hunt, J.M., Anal. Biochem. 16: 349 (1966).
McMahon, R.E., J. Pharm. Sci. 55: 457 (1966).
Tolbert, B.M., and Cozzetto, F. J., in: Preparation and Bio-Medical Application of Labeled Molecules, Euratom, 1964.
Okita, G.T., in: Isotopes in Experimental Pharmacology, University of Chicago Press, Chicago, 1965.
Way, E.L., and Adler, T.K., The Biological Disposition of Morphine and Its Surrogates, World Health Organization, Geneva, 1960.
Welles, J.S., McMahon, R.E., and Doran, W. J., J. Pharmacol. 139: 166 (1963).
Ober, R.E., J. Labeled Compds. 2: 203 (1966).
Rosenblum, C., in: Isotopes in Experimental Pharmacology, University of Chicago Press, Chicago, 1965.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1968 New England Nuclear Corporation
About this chapter
Cite this chapter
McMahon, R.E., Marshall, F.J. (1968). The Preparation of N-Methyl-C14-Labeled Drugs. In: Rothchild, S. (eds) Advances in Tracer Methodology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-7532-6_3
Download citation
DOI: https://doi.org/10.1007/978-1-4684-7532-6_3
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-7534-0
Online ISBN: 978-1-4684-7532-6
eBook Packages: Springer Book Archive