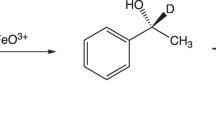
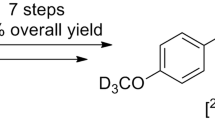
A deuterated drug comprises diminutive molecules in which deuterium replaces one or more than one hydrogen atoms of the molecule. As deuterium and hydrogen possess almost similar physical properties, deuterium exchange is the smallest structural change that can be made to a molecule. This process is called deuteration. It is an extremely useful tool for the enrichment of drug’s metabolism. Selective replacement with deuterium leads to amplified bond strength which in turn increases the biological half-life and thus metabolic stability of the drug. Furthermore, deuterium substitution may also result in metabolic shunting leading to decreased exposure of critical organs to unwanted and toxic metabolites or increased exposure to desired active metabolites. This article focuses on numerous illustrations where deuteration came up with improvement of metabolic stability of drug and reduction in toxicity relative to the untreated drug while retaining its substantial pharmacological profile. Deuterated edition of present drugs can demonstrate better pharmacokinetic or toxicological properties due to stronger deuterium–carbon bonds by altering their metabolism and hence are a subject of major research.








Similar content being viewed by others
References
S. Kaur and M. Gupta, Glob. J. Pharmaceut. Sci., 1(4), (2017).
A. Zlatska, I. Gordiienko, R. Vasyliev, et al., The Scientific World J., Vol. 2018, Article ID 5454367, 10 pages (2018).
C. R. Carr, A. Taheri, and L. A. Berben, J. Am. Chem. Soc., 142(28), 12299 – 12305 (2020).
F. H. Westheimer, Chem. Rev., 61(3), 265 – 273 (1961).
Z. Mao and C. Campbell, ACS Catalysis, 10(7), 4181 – 4192 (2020).
G. Timmins, Expert Opin. Ther. Pat., 24(10), 1067 – 1075 (2014).
R. H. Howland, J. Psychosocial Nursing and Mental Health Services, 53(9), 13 – 16 (2015).
T. Pirali, M. Serafini, S. Cargnin, and A. Genazzani, J. Med. Chem., 62(11), 5276 – 5297 (2019).
S. L. Harbeson and R. D. Tung, Annu. Rep. Med. Chem., 46, 403 – 417 (2011).
M. Dean and V. Sung, Drug Des. Devel. Ther., 12, 313 – 319 (2018).
F. Maltais, Y. Jung, M. Chen, et al., J. Med. Chem., 52(24), 7993 – 8001 (2009).
R. Vardanyan and V. H. Ruby, Synthesis of Best-Seller Drugs, 687 – 736 (2016).
R. Garay and G. Grossberg, Expert Opin. Investig. Drugs, 26(1), 121 – 132 (2017).
J. L. Cummings, C. G. Lyketsos, E. R. Peskind, et al., JAMA, 314(12), 1242 – 1254 (2015).
L. Wu, A. Aslanian, J. Liu, et al., Blood, 120(21), 2463 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, N., Kini, R. & Kad, P. Deuterated Drugs. Pharm Chem J 55, 1372–1377 (2022). https://doi.org/10.1007/s11094-022-02584-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02584-4