Abstract
Ectopic pregnancy is identified with the widely-applied assisted reproductive technology (ART). Bilateral ectopic pregnancy is a rare form of ectopic pregnancy which is difficult to be diagnosed at the pre-operation stage. In this paper, we presented an unusual case of heterochronic bilateral ectopic pregnancy after stimulated intrauterine insemination (IUI), where there has been a delay of 22 d between the diagnoses of the two ectopic pregnancies. Literature was reviewed on the occurrence of bilateral ectopic pregnancy during the past four years in the MEDLINE database. We found 16 cases of bilateral ectopic pregnancy reported since 2008, and analyzed the characteristics of those cases of bilateral ectopic pregnancy. We emphasize that ovulation induction and other ARTs may increase the risk of bilateral ectopic pregnancy. Because of the difficulty in identification of bilateral ectopic pregnancy by ultrasonography, the clinician should be aware that the treatment of one ectopic pregnancy does not preclude the occurrence of a second ectopic pregnancy in the same patient and should pay attention to the intra-operation inspection of both side fallopian tubes in any ectopic pregnancy case.
概要
研究目的
报道一例不同步双侧异位妊娠, 并回顾相关文献, 总结规律。
创新要点
第一次报道了不同步的双侧异位妊娠。
研究方法
对促排卵后行宫腔内人工授精病人发生非同步双侧输卵管妊娠的病例进行报告, 辅以人绒毛膜促性腺激素 (hCG) 值波动曲线、 超声图像以及病理切片加以阐述; 同时回顾 2008 年以来关于双侧异位妊娠的文献, 并分析病例特征。
重要结论
促排卵后可能会提高双侧异位妊娠风险, 一侧异位妊娠发生后, 需要注意对侧是否也存在异位妊娠。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ectopic pregnancy is an important cause of maternal morbidity and mortality where implantation of a fertilized ovum occurs outside the uterine cavity. Approximately 1%–2% of all reported pregnancies are ectopic pregnancies (Farquhar, 2005) in the USA, as well as in China. Some risk factors of ectopic pregnancies including previous ectopic pregnancy, genital infection, pelvic inflammatory disease (PID), tubal disease or surgery, and smoking have been clarified (Farquhar, 2005). Assisted reproductive technology (ART) is a known risk factor for ectopic pregnancy. The ectopic pregnancy rate is higher in pregnancies resulting from ART than in spontaneous pregnancies, with the incidence ranging from 2.1% to 8.6%, especially ingamete and zygote intrafallopian transfer (GIFT and ZIFT) (Nazari et al., 1993; Clayton et al., 2006).
In our study, we describe a case of a bilateral ectopic pregnancy, where the timing of the diagnosis via laparoscopy was 22 d apart in a patient who had previously undergone ovulation induction and intrauterine insemination (IUI).
2 Case report and literature review
A 25-year-old woman with a history of subfertility for three years, attended the Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, China. She had a previous history of abortion and polycystic ovary syndrome (PCOS) diagnosed six months ago. She had oligomenorrhea and ultrasound features of polycystic ovaries. The basal endocrinal results at cycle Day 3 were as follows: luteinizing hormone (LH) 6.62 IU/L, follicle-stimulating hormone (FSH) 4.71 IU/L, testosterone (TTE) 2.8 nmol/L, oestrodial (E2) 139.9 pmol/L, progesterone (P) 3.77 nmol/L, and prolactin (PRL) 15.6 ng/ml. The hysterosal**ography (HSG) showed that the uterine cavity and fallopian tubes were spreading out. The semen analysis of her husband was normal according to the criteria from the World Health Organization (WHO).
The patient underwent two cycles of ovulation induction and one failed cycle of stimulated IUI, but conceived on the third cycle of ovulation induction. She had three follicles over 17 mm and was triggered with 10 000 IU human chorionic gonadotrophin (hCG).
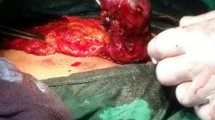
On Day 15 of post-IUI, the woman was admitted to the emergency room because of light vaginal bleeding. Her serum β-hCG was 60.23IU/L (Fig. 1). Eight days later, the patient was admitted complaining of right lower abdominal pain with β-hCG 1204IU/L, and transvaginal ultrasonography (USG) revealed a possible right adnexal mass suggestive of an ectopic pregnancy lesion (Figs. 2a and 2b). The patient had a laparoscopy and right sal**ectomy for a 4-cm unruptured right ampullary ectopic pregnancy. The left fallopian tube and the rest of the pelvis were inspected and deemed normal. Histological examination confirmed the presence of the early placenta in the resected right tube (Fig. 3a). Serum β-hCG level dropped to 377.9IU/L on the first day after the surgery. The patient was discharged 3 d later.
Pelvic ultrasonography (USG) images
(a) The uterus showed the absence of any gestation sac. No pseudosac was detected before the first surgery. (b) Ultrasound revealed the right ovary and the lateral mass, in which a gestational sac could be seen. (c, d) Ultrasound showed bilateral ovaries on Day 19 after the first surgery of the right ectopic pregnancy: the left ovary (c) and the right ovary (d). (e, f) Ultrasound results on Day 21 after the first surgery of the ectopic pregnancy: the normal uterus and endometrium (e) and a mass adjacent to but separated from the left ovary (f). (g) A large mass was detected by vaginal ultrasound in the left side. (h) A large collection of hemorrhagic fluid in the pelvic cavity
The patient was monitored in accordance to local protocol because her serum β-hCG started to increase again after an initial decrease, and a USG performed 21 d after the first surgery revealed a complex mass measuring 2.2cm×1.4cm×1.6cm in the left adnexa (Figs. 2c–2f). As the serumβ-hCG level (1049IU/L) was relatively low, methotrexate treatment was administrated intramuscularly after obtaining an informed consent signed by the patient.
Three days after the methotrexate administration, the patient suffered acutely from severe left lower quadrant pain, tachycardia (110 beats/min), and hypotension (76/49 mmHg), with signs of acute blood loss and the hemoglobin concentration dropped from 13.3 to 11.9 g/dl within 1 h. USG revealed a 3.7 cm×2.8 cm×2.7 cm left adnexal mass suggestive of an ectopic pregnancy and free fluid was also noted around the left fallopian tube (Figs. 2g and 2h). She had a left sal**ectomy, and the histological examination confirmed the left ectopic pregnancy (Fig. 3b).
For the latest information on bilateral EP, we searched the MEDLINE database for case reports since 2008 using the terms “bilateral ectopic pregnancy” and “bilateral tubal pregnancy”, and obtained 16 reports in English (Table 1). Of the 16 reports of bilateral ectopic pregnancy, 14 were tubal ectopic pregnancies, one was a bilateral ovarian ectopic pregnancy, and the other one was a bilateral interstitial ectopic pregnancy. In the 16 cases, 8 (50%) bilateral ectopic pregnancies occurred spontaneously, 7 (43%) cases were the result of ART including ovulation induction, and in one case, the author did not mention the origin of the condition.
3 Discussion
Bilateral ectopic pregnancy is the rarest ectopic pregnancy with its incidence oscillating between 1/725 and 1/1580 for all ectopic pregnancies (Abrams and Kanter, 1948; Stewart, 1950; de los Ríos et al., 2007). Multiple ovulations after ovulation induction easily lead to multiple pregnancies including bilateral ectopic pregnancies. Most of the previous articles on bilateral ectopic pregnancy also stated that it was the result of the use of ART. de los Ríos et al. (2007) reviewed 42 cases of bilateral ectopic pregnancy between 1997 and 2007, of which 19 (45%) were the result of ART.
In our literature review of the 16 reports of bilateral ectopic pregnancies in the most recent four years, one was a bilateral ovarian ectopic pregnancy and one was a bilateral interstitial ectopic pregnancy. According to the existing epidemiological data, ovarian pregnancies and interstitial pregnancies occurred in 3.0% and 2.5% of all ectopic pregnancies (Bouyer et al., 2002). Given the limited sample size of our literature review, the frequency of these two rare ectopic pregnancies could be considered consistent with previous research, and needs to be further verified.
Half of the 16 bilateral ectopic pregnancy cases in the literature review occurred spontaneously, while 7 (43%) cases were related to ovulation induction. Two previous reviews showed that the spontaneous bilateral ectopic pregnancy was responsible for 21 of 42 (50%) (de los Ríos et al., 2007) and 14 of 38 (36%) (Bustos Lopez et al., 1998) between 1997–2007 and 1980–1997 of all bilateral ectopic pregnancies, respectively. Taking into account all the cases reported since 1980, we might conclude that in the last 31 years, 44% of the reported bilateral ectopic pregnancies had been spontaneous.
The discussion on whether ART is a risk factor of ectopic pregnancy still continues. The risk of ectopic pregnancy associated with ART has been discussed in many studies (Clayton et al., 2006; Chang and Suh, 2010). Ectopic risk varies according to the reproductive health characteristics of women and the ART procedure type. Clayton et al. (2006) claimed that ART procedures increased the overall ectopic pregnancy rate. The most reasonable explanation was that ART patients with tubal factor infertility contributed to the higher ectopic pregnancy rate due to their impaired tubal function, but tubal infertility was not the only reason. Patients with non-tubal female factors of infertility also conceived more ectopically (odds ratio (OR) 1.4, 95% confidence interval (CI) 1.2–1.6) (Clayton et al., 2006). However, most existing researches still have limitations such as failing to rule out the impact of the infertile background of women who had ART. A tubal factor (sal**itis, tubal surgery, sterilization, previous ectopic pregnancy) is a well-known condition for ectopic pregnancy with an infertile background. Therefore, some researchers, like de los Ríos et al. (2007), doubted the conclusion that ART promoted ectopic pregnancies.
Studies have shown that ovulation induction, especially with clomiphene citrate (CC), was an independent risk factor of ectopic pregnancy (Marchbanks et al., 1985; Cohen et al., 1986; Verhulst et al., 1993), and in vitro fertilization (IVF) did not increase the risk further (Fernandez et al., 1991). In the cases we reviewed, two were reported after CC induction (Yu et al., 2008; Jeong et al., 2009). However, protocols using gonadotropin-releasing hormone analogues (GnRHa) did not show any increased risk (Verhulst et al., 1993). Compared with matched controls with intrauterine pregnancies, the ectopic pregnancy group had significantly higher peak oestradiol levels (Karande et al., 1991). Techniques applied in the fallopian tubes, such as ZIFT and GIFT, significantly increased the rate of ectopic pregnancies (Slowey and Scoccia, 1993; Clayton et al., 2006).
Among the 16 cases, the ectopic pregnancy on both two sides was identified by USG in only 6 cases, one by a computer tomography (CT) scan, and the others by exploratory laparoscopy. In de los Ríos et al. (2007)’s review, only 2 of 42 cases were recognized and accurately diagnosed by USG. This poor rate of diagnosis by ultrasound revealed the fact that USG might not be very helpful in the identication of bilateral ectopic pregnancies. Therefore, the combination of symptoms, serum β-hCG level measurement, and USG should all be considered before operating. The inspection of both fallopian tubes should not be forgotten during the surgery in order to prevent a worsening prognosis, or even a life-threatening condition (Ayoubi and Fanchin, 2003).
The most unusual part of this case is the heterochronism of the bilateral ectopic pregnancy. Most reported bilateral ectopic pregnancies occurred and developed simultaneously, so the diagnoses were made at the same time on both sides. In this case, careful exploration of the contralateral fallopian tube did not reveal any signs of a develo** ectopic pregnancy. The first and second ectopic pregnancies were diagnosed 23 and 45 d, respectively, after the original IUI. The two embryos were either derived from two fertilized ovums or one cleaved blastocyst. The rates of development of the two blastocysts could be distinctly different, as an observation that is not uncommon in the IVF laboratory during the early stages of embryo development, thus accounting for the delay in diagnosis between the two ectopic pregnancies. At the first exploration, the contralateral implanted embryo was too underdeveloped to be spotted by laparoscopy, which contributed to the consequently delayed diagnosis of ectopic pregnancy. IVF or intracytoplasmic sperm injection (ICSI) following ovulation induction has shown adverse effect on offspring, and this study showed the association of ovulation with bilateral ectopic pregnancy (Wang et al., 2013; **ng et al., 2014).
In this paper, we present an unusual case of bilateral ectopic pregnancy after stimulated IUI, where there has been a substantial delay of 22 d between the diagnoses of the two sides of the ectopic pregnancies. This paper highlights to clinicians the importance that bilateral ectopic pregnancies can arise after assisted conception and that diagnosis and treatment of one ectopic pregnancy do not preclude the occurrence of a second ectopic pregnancy in the same patient, especially if she continues to have rising β-hCG and persistent symptoms.
Compliance with ethics guidelines
Bo ZHU, Gu-feng XU, Yi-feng LIU, Fan QU, Wei-miao YAO, Yi-min ZHU, Hui-juan GAO, and Dan ZHANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
Abrams, R.A., Kanter, A.E., 1948. Bilateral simultaneous extrauterine pregnancy. Am. J. Obstet. Gynecol., 56(6): 1198–1200.
Altinkaya, S.O., Ozat, M., Pektas, M.K., et al., 2008. Simultaneous bilateral tubal pregnancy after in vitro fertilization and embryo transfer. Taiwan. J. Obstet. Gynecol., 47(3):338–340. [doi:10.1016/S1028-4559(08)60136-9]
Andrews, J., Farrell, S., 2008. Spontaneous bilateral tubal pregnancies: a case report. J. Obstet. Gynaecol. Can., 30(1):51–54.
Ayoubi, J.M., Fanchin, R., 2003. Ectopic pregnancy: which side to operate? Lancet, 362(9391):1183. [doi:10.1016/S0140-6736(03)14540-0]
Bouyer, J., Coste, J., Fernandez, H., et al., 2002. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum. Reprod., 17(12):3224–3230. [doi:10.1093/humrep/17.12.3224]
Bustos Lopez, H.H., Rojas-Poceros, G., Barron Vallejo, J., et al., 1998. Conservative laparoscopic treatment of bilateral ectopic pregnancy: 2 case reports and review of the literature. Ginecol. Obstet. Mex., 66:13–17.
Chang, H.J., Suh, C.S., 2010. Ectopic pregnancy after assisted reproductive technology: what are the risk factors? Curr. Opin. Obstet. Gynecol., 22(3):202–207. [doi:10.1097/GCO.0b013e32833848fd]
Clayton, H.B., Schieve, L.A., Peterson, H.B., et al., 2006. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet. Gynecol., 107(3):595–604. [doi:10.1097/01.AOG.0000196503.78126.62]
Cohen, J., Mayaux, M.J., Guihard-Moscato, M.L., et al., 1986. In-vitro fertilization and embryo transfer: a collaborative study of 1163 pregnancies on the incidence and risk factors of ectopic pregnancies. Hum. Reprod., 1(4):255–258.
de los Ríos, J.F., Castañeda, J.D., Miryam, A., 2007. Bilateral ectopic pregnancy. J. Minim. Invasive Gynecol., 14(4): 419–427. [doi:10.1016/j.jmig.2007.01.011]
El Hakim, E., Cahill, D., 2009. Concurrent bilateral ectopic pregnancy after recurrent miscarriages in a fertile woman. J. Obstet. Gynaecol., 29(4):359. [doi:10.1080/014436 10902838373]
Farquhar, C.M., 2005. Ectopic pregnancy. Lancet, 366(9485): 583–591. [doi:10.1016/S0140-6736(05)67103-6]
Fernandez, H., Coste, J., Job-Spira, N., 1991. Controlled ovarian hyperstimulation as a risk factor for ectopic pregnancy. Obstet. Gynecol., 78(4):656–659.
Ghaffari, F., Eftekhari Yazdi, P., Kiani, K., 2011. A case report of bilateral tubal ectopic pregnancy following day 5 embryo transfer. Arch. Med. Sci., 7(6):1087–1088. [doi:10.5114/aoms.2011.26626]
Greenberg, J.A., 2008. Bilateral ectopic pregnancy. Rev. Obstet. Gynecol., 1(2):48.
Issat, T., Grzybowski, W., Jakimiuk, A.J., 2009. Bilateral ectopic tubal pregnancy, following in vitro fertilisation (IVF). Folia Histochem. Cytobiol., 47(5):S147–S148.
Jeong, H.C., Park, I.H., Yoon, Y.S., et al., 2009. Heterotopic triplet pregnancy with bilateral tubal and intrauterine pregnancy after spontaneous conception. Eur. J. Obstet. Gynecol. Reprod. Biol., 142(2):161–162. [doi:10.1016/j.ejogrb.2008.10.013]
Karande, V.C., Flood, J.T., Heard, N., et al., 1991. Analysis of ectopic pregnancies resulting from in-vitro fertilization and embryo transfer. Hum. Reprod., 6(3):446–449.
Liao, C.Y., Ding, D.C., 2009. Laparoscopic management of spontaneous bilateral tubal pregnancies. J. Minim. Invasive Gynecol., 16(3):247. [doi:10.1016/j.jmig.2008.09.610]
Marasinghe, J.P., Condous, G., Amarasinghe, W.I., 2009. Spontaneous bilateral tubal ectopic pregnancy. Ceylon Med. J., 54(1):21–22. [doi:10.4038/cmj.v54i1.470]
Marchbanks, P.A., Coulam, C.B., Annegers, J.F., 1985. An association between clomiphene citrate and ectopic pregnancy: a preliminary report. Fertil. Steril., 44(2): 268–270.
Martinez, J., Cabistany, A.C., Gonzalez, M., et al., 2009. Bilateral simultaneous ectopic pregnancy. South. Med. J., 102(10):1055–1057. [doi:10.1097/SMJ.0b013e3181b67378]
Nazari, A., Askari, H.A., Check, J.H., et al., 1993. Embryo transfer technique as a cause of ectopic pregnancy in in vitro fertilization. Fertil. Steril., 60(5):919–921.
Pan, J., Qian, Y., Wang, J., 2010. Bilateral interstitial pregnancy after in vitro fertilization and embryo transfer with bilateral fallopian tube resection detected by transvaginal sonography. J. Ultrasound Med., 29(12):1829–1832.
Plotti, F., di Giovanni, A., Oliva, C., et al., 2008. Bilateral ovarian pregnancy after intrauterine insemination and controlled ovarian stimulation. Fertil. Steril., 90(5): 2015.e3–2015.e5. [doi:10.1016/j.fertnstert.2008.02.117]
Sentilhes, L., Bouet, P.E., Jalle, T., et al., 2009. Ultrasound diagnosis of spontaneous bilateral tubal pregnancy. Aust. N. Z. J. Obstet. Gynaecol., 49(6):695–696. [doi:10.1111/j.1479-828X.2009.01081.x]
Shetty, J.P., Shetty, B., Makkanavar, J.H., et al., 2011. A rare case of bilateral tubal pregnancy. J. Indian Med. Assoc., 109(7):506–507.
Slowey, M.J., Scoccia, B., 1993. Simultaneous bilateral ectopic pregnancy resulting from gamete intrafallopian transfer (gift). J. Assist. Reprod. Genet., 10(4):304–308. [doi:10.1007/BF01204947]
Stewart, H.L.Jr., 1950. Bilateral ectopic pregnancy. West J. Surg. Obstet. Gynecol., 58(11):648–656.
Verhulst, G., Camus, M., Bollen, N., et al., 1993. Analysis of the risk factors with regard to the occurrence of ectopic pregnancy after medically assisted procreation. Hum. Reprod., 8(8):1284–1287.
Wali, A.S., Khan, R.S., 2012. Spontaneous bilateral tubal pregnancy. J. Coll. Physicians Surg. Pak., 22(2):118–119.
Wang, L.Y., Wang, N., Le, F., et al., 2013. Persistence and intergenerational transmission of differentially expressed genes in the testes of intracytoplasmic sperm injection conceived mice. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 14(5):372–381. [doi:10.1631/jzus.B1200321]
**ng, L.F., Qian, Y.L., Chen, L.T., et al., 2014. Is there a difference in cognitive development between preschool singletons and twins born after intracytoplasmic sperm injection or in vitro fertilization? J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 15(1):51–57. [doi:10.1631/jzus. B1300229]
Yu, H.T., Huang, H.Y., Lai, C.H., et al., 2008. Conservative laparoscopy following prophylactic methotrexate for an unruptured bilateral tubal pregnancy. Taiwan. J. Obstet. Gynecol., 47(4):451–453. [doi:10.1016/S1028-4559(09)60017-6]
Author information
Authors and Affiliations
Corresponding author
Additional information
The two authors contributed equally to this work Project supported by the National Basic Research Program (973) of China (No. 2013CB967404), the National Natural Science Foundation of China (Nos. 81170310 and 81270664), the Science Foundation for Distinguished Young Scholars of Zhejiang Province (No. LR14H040001), and the Talent Project of Zhejiang Province (No. 2011RCA028), China
Rights and permissions
About this article
Cite this article
Zhu, B., Xu, Gf., Liu, Yf. et al. Heterochronic bilateral ectopic pregnancy after ovulation induction. J. Zhejiang Univ. Sci. B 15, 750–755 (2014). https://doi.org/10.1631/jzus.B1400081
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1400081
Key words
- Heterochronic bilateral ectopic pregnancy
- Assisted reproductive technology
- Intrauterine insemination, Ovulation induction