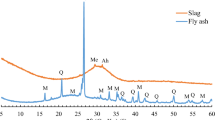
Abstract
The effect of ambient temperature on the engineering properties of alkali-activated materials (AAM) needs to be further investigated due to the high variety of activating solutions in the AAM technology. This paper presents the rheological behavior, structural build-up, reaction kinetics and mechanical properties of GGBFS-FA mixtures activated by sodium hydroxide, sodium silicate, sodium carbonate and sodium sulfateinvestigated under different ambient temperature conditions. It was found that the effect of ambient temperature on the rheology and reaction kinetics was highly dependent on the activator type. Under room temperature conditions, the highest and lowest yield stress values were obtained in sodium hydroxide and sodium silicate mixtures, respectively. The increase in temperature did not affect the yield stresses and viscosities of sodium carbonate and sodium sulfate mixtures; however, the yield stresses of sodium hydroxide and sodium silicate mixtures significantly increased. This effect was more pronounced in mixtures with high Ms values. Higher storage modulus values were obtained with an increase in temperature, indicating initial structuration with temperature. The increasing temperature enhanced the compressive strength of alkali-activated GGBFS-FA mixtures. This improvement was more pronounced at early ages for the sodium silicate mixture, and at later ages for the sodium carbonate and sodium sulfate mixtures, while it was very limited in the sodium hydroxide mixture. The SEM images and calorimetric measurements showed the formation of a denser microstructure and enhancement in the exothermic peak with a shorter induction period with an increase in temperature.









Similar content being viewed by others
References
Provis JL (2014) Geopolymers and other alkali activated materials: why how and what. Mater Struct. https://doi.org/10.1617/s11527-013-0211-5
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, Van Deventer JSJ (2007) Geopolymer technology: the current state of the art. J Mater Sci 42(9):2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Palomo A, Krivenko P, Garcia-Lodeiro I, Kavalerova E, Maltseva O, Fernández-Jiménez A (2014) A review on alkaline activation: new analytical perspectives. Materiales de Construccion. https://doi.org/10.3989/mc.2014.00314
Provis JL, van Deventer JSJ (Eds) (2014) Alkali-Activated materials: State-of-the-Art Report, RILEM TC 224-AAM, Springer/RILEM, Dordrecht, 11. https://doi.org/10.1007/978-94-007-7672-2.
van Deventer JSJ, Nicolas RS, Ismail I, Bernal SA, Brice DG, Provis JL (2014) Microstructure and durability of alkali-activated materials as key parameters for standardization. J Sustain Cem Based Mater 4(2):116–128. https://doi.org/10.1080/21650373.2014.979265
Behfarnia K, Rostami M (2017) An assessment on parameters affecting the carbonation of alkali-activated slag concrete. J Clean Prod 157:1–9. https://doi.org/10.1016/j.jclepro.2017.04.097
Yang KH, Song JK, Song K (2013) Assessment of CO 2 reduction of alkali-activated concrete. J Clean Prod 39:265–272. https://doi.org/10.1016/j.jclepro.2012.08.001
Yang KH, Song JK, Song K (2013) Assessment of CO2 reduction of alkali-activated concrete. J Clean Prod 39:265–272. https://doi.org/10.1016/j.jclepro.2012.08.001
Higgins D (2007) Briefing: GGBS and sustainability. Proc Inst Civ Eng Constr Mater 160(3):99–101. https://doi.org/10.1680/coma.2007.160.3.99
Shi C, Qu B, Provis JL (2019) Recent progress in low-carbon binders. Cem Concr Res 122(May):227–250. https://doi.org/10.1016/j.cemconres.2019.05.009
Kashani A (2022) Influence of precursors on rheology of alkali-activated materials. LTD. https://doi.org/10.1016/b978-0-323-85469-6.00004-0
Dai X, Aydın S, Yardımcı MY, Lesage K, De Schutter G (2020) Effects of activator properties and GGBFS/FA ratio on the structural build-up and rheology of AAC. Cem Concr Res 138:106253. https://doi.org/10.1016/j.cemconres.2020.106253
Dai X, Aydin S, Yardimci MY, De Schutter G (2022) Rheology and structural build-up of sodium silicate- and sodium hydroxide-activated GGBFS mixtures. Cem Concr Compos. https://doi.org/10.1016/j.cemconcomp.2022.104570
Dai X, Tao Y, Van Tittelboom K, De Schutter G (2023) Rheological and mechanical properties of 3D printable alkali-activated slag mixtures with addition of nano clay. Cem Concr Compos. https://doi.org/10.1016/j.cemconcomp.2023.104995
Palacios M, Banfill PFG, Puertas F (2008) Rheology and setting of alkali-activated slag pastes and mortars: effect of organic admixture. ACI Mater J 105(2):140–148. https://doi.org/10.14359/19754
Puertas F, Varga C, Alonso MM (2014) Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem Concr Compos 53:279–288. https://doi.org/10.1016/j.cemconcomp.2014.07.012
Poulesquen A, Frizon F, Lambertin D (2013) Rheological behavior of alkali-activated metakaolin during geopolymerization. Cem Mater Nucl Waste Storage 357(21):225–238. https://doi.org/10.1007/978-1-4614-3445-0_20
Rifaai Y, Yahia A, Mostafa A, Aggoun S, Kadri EH (2019) Rheology of fly ash-based geopolymer: Effect of NaOH concentration. Constr Build Mater 223:583–594. https://doi.org/10.1016/j.conbuildmat.2019.07.028
Zhao Y, Ma Z, Qiu J, Sun X, Gu X (2021) Temperature-dependent hydration and mechanical properties of high-volume fly ash cement with chemical additives. J Mater Civ Eng 33(5):04021065. https://doi.org/10.1061/(asce)mt.1943-5533.0003684
Rivera OG et al (2016) Effect of elevated temperature on alkali-activated geopolymeric binders compared to Portland cement-based binders. Cem Concr Res 90:43–51. https://doi.org/10.1016/j.cemconres.2016.09.013
Fan Y, Yin S, Wen Z, Zhong J (1999) Activation of fly ash and its effects on cement properties. Cem Concr Res 29(4):467–472. https://doi.org/10.1016/S0008-8846(98)00178-1
Puertas F, Martínez-Ramírez S, Alonso S, Vázquez T (2000) Alkali-activated fly ash/slag cements. Strength behaviour and hydration products. Cem Concr Res 30(10):1625–1632. https://doi.org/10.1016/S0008-8846(00)00298-2
Mehdizadeh H, Kani EN (2018) Rheology and apparent activation energy of alkali activated phosphorous slag. Constr Build Mater 171:197–204. https://doi.org/10.1016/j.conbuildmat.2018.03.130
Palacios M, Alonso MM, Varga C, Puertas F (2019) Influence of the alkaline solution and temperature on the rheology and reactivity of alkali-activated fly ash pastes. Cem Concr Compos 95:277–284. https://doi.org/10.1016/j.cemconcomp.2018.08.010
Lu C, Zhang Z, Hu J, Yu Q, Shi C (2022) Relationship between rheological property and early age-microstructure building up of alkali-activated slag. Compos B Eng 247:110271. https://doi.org/10.1016/j.compositesb.2022.110271
Chithiraputhiran S, Neithalath N (2013) Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr Build Mater 45:233–242. https://doi.org/10.1016/j.conbuildmat.2013.03.061
Yahia A, Khayat KH (2001) Analytical models for estimating yield stress of high-performance pseudoplastic grout. Cem Concr Res 31(5):731–738
Feys D, Wallevik JE, Yahia A, Khayat KH, Wallevik OH (2013) Extension of the Reiner–Riwlin equation to determine modified Bingham parameters measured in coaxial cylinders rheometers. Mater Struct. https://doi.org/10.1617/s11527-012-9902-6
Yuan Q, Zhou D, Khayat KH, Feys D, Shi C (2017) On the measurement of evolution of structural build-up of cement paste with time by static yield stress test vs. small amplitude oscillatory shear test. Cem Concr Res 99:183–189. https://doi.org/10.1016/j.cemconres.2017.05.014
European committee for standardization, (2019) EN 1015–11:Methods of test for mortar for masonry-part 11: Determination of flexural and compressive strength of hardened mortar
Snellings R, Chwast J, Cizer Ö, De Belie N, Dhandapani Y, Durdzinski P, Elsen J et al (2019) R TC-238“RILEM TC - 238 SCM Recommendation on hydration stoppage by solvent exchange for the study of hydrate assemblages.” Mater Struct. https://doi.org/10.1617/s11527-018-1298-5
Favier A, Hot J, Habert G, Roussel N, D’Espinose De Lacaillerie JB (2014) Flow properties of MK-based geopolymer pastes. A comparative study with standard Portland cement pastes. Soft Matter 10(8):1134–1141. https://doi.org/10.1039/c3sm51889b
Alnahhal MF, Kim T, Hajimohammadi A (2021) Distinctive rheological and temporal viscoelastic behaviour of alkali-activated fly ash/slag pastes: a comparative study with cement paste. Cem Concr Res 144:106441. https://doi.org/10.1016/j.cemconres.2021.106441
Vance K, Dakhane A, Sant G, Neithalath N (2014) Observations on the rheological response of alkali activated fly ash suspensions: the role of activator type and concentration. Rheol Acta 53(10–11):843–855. https://doi.org/10.1007/s00397-014-0793-z
Roussel N, Lemaître A, Flatt RJ, Coussot P (2010) Steady state flow of cement suspensions: a micromechanical state of the art. Cem Concr Res 40(1):77–84. https://doi.org/10.1016/j.cemconres.2009.08.026
Kashani A, Provis JL, Qiao GG, Van Deventer JSJ (2014) The interrelationship between surface chemistry and rheology in alkali activated slag paste. Constr Build Mater 65:583–591. https://doi.org/10.1016/j.conbuildmat.2014.04.127
Gebregziabiher BS, Thomas R, Peethamparan S (2015) Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem Concr Compos 55:91–102. https://doi.org/10.1016/j.cemconcomp.2014.09.001
Yang X, Zhu W, Yang Q (2008) The viscosity properties of sodium silicate solutions. J Solut Chem 37(1):73–83. https://doi.org/10.1007/s10953-007-9214-6
Vázquez G, Alvarez E, Varela R, Cancela A, Navaza JM (1996) Density and viscosity of aqueous solutions of sodium dithionite, sodium hydroxide, sodium dithionite + sucrose, and sodium dithionite + sodium hydroxide + sucrose from 25°C to 40°C. J Chem Eng Data 41(2):244–248. https://doi.org/10.1021/je950243k
Roussel N, Ovarlez G, Garraul S, Brumaud C (2012) The origins of thixotropy of fresh cement pastes. Cem Concr Res 42:148–157. https://doi.org/10.1016/j.cemconres.2011.09.004
Flatt RJ, Bowen P (2003) Electrostatic repulsion between particles in cement suspensions: domain of validity of linearized Poisson-Boltzmann equation for nonideal electrolytes. Cem Concr Res 33(6):781–791. https://doi.org/10.1016/S0008-8846(02)01059-1
Onisei S, Lesage K, Blanpain B, Pontikes Y (2015) Early age microstructural transformations of an inorganic polymer made of fayalite slag. J Am Ceram Soc 98(7):2269–2277. https://doi.org/10.1111/jace.13548
Dai X, Aydin S, Yardımcı MY, Qiang R, Lesage K, De Schutter G (2021) Rheology, early-age hydration and microstructure of alkali-activated GGBFS-Fly ash-limestone mixtures. Cem Concr Compos 124:104244. https://doi.org/10.1016/j.cemconcomp.2021.104244
Dai X, Aydın S, Yardımcı MY, Lesage K, De Schutter G (2022) Rheology and microstructure of alkali-activated slag cements produced with silica fume activator. Cem Concr Compos 125:104303. https://doi.org/10.1016/j.cemconcomp.2021.104303
Aupoil J, Champenois JB, de d’Espinose Lacaillerie JB, Poulesquen A (2018) Interplay between silicate and hydroxide ions during geopolymerization. Cem Concr Res 115:426–432. https://doi.org/10.1016/j.cemconres.2018.09.012
Dai X, Ren Q, Aydin S, Yardımcı MY, Lesage K, De Schutter G (2021) Enhancing thixotropy and structural build-up of alkali- activated slag/fly ash pastes with nano clay. Mater Struct. https://doi.org/10.1617/s11527-021-01760-4
Rouyer J, Poulesquen A (2015) Evidence of a fractal percolating network during geopolymerization. Am Ceram Soc. https://doi.org/10.1111/jace.13480
Steins P, Poulesquen A, Diat O, Frizon F (2012) Structural evolution during geopolymerization from an early age to consolidated material. Langmuir 28(22):8502–8510. https://doi.org/10.1021/la300868v
Siddique S, Gupta V, Chaudhary S, Park S, Jang JG (2021) Influence of the precursor, molarity and temperature on the rheology and structural buildup of alkali-activated materials. Materials. https://doi.org/10.3390/ma14133590
ThomaTs JJ (2012) The instantaneous apparent activation energy of cement hydration measured using a novel calorimetry-based method. J Am Ceram Soc 95(10):3291–3296. https://doi.org/10.1111/j.1551-2916.2012.05396.x
Gao X, Yu QL, Brouwers HJH (2015) Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr Build Mater 80:105–115. https://doi.org/10.1016/j.conbuildmat.2015.01.065
Dai X, Aydin S, Yardımcı MY, Lesage K, De Schutter G (2020) Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr Build Mater 264:120253. https://doi.org/10.1016/j.conbuildmat.2020.120253
Sun Z, Vollpracht A (2018) Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem Concr Res 103:110–122. https://doi.org/10.1016/j.cemconres.2017.10.004
Gebregziabiher BS, Thomas RJ, Peethamparan S (2016) Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr Build Mater 113:783–793. https://doi.org/10.1016/j.conbuildmat.2016.03.098
AlMakhadmeh W, Soliman A (2020) Effect of activator nature on property development of alkali-activated slag binders. J Sustain Cem Based Mater 10(4):240–256
Gao X, Yu QL, Brouwers HJH (2015) Properties of alkali activated slag-fly ash blends with limestone addition. Cem Concr Compos 59:119–128. https://doi.org/10.1016/j.cemconcomp.2015.01.007
Shi C, Day RL (1995) A calorimetric study of early hydration of alkali-slag cements. Cem Concr Res 25(6):1333–1346
Brough AR, Atkinson A (2002) Sodium silicate-based, alkali-activated slag mortars-part I. Strength, hydration and microstructure. Cem Concr Res 32(6):865–879. https://doi.org/10.1016/S0008-8846(02)00717-2
Puertas F, Fernández-Jiménez A, Blanco-Varela MT (2004) Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem Concr Res 34(1):139–148. https://doi.org/10.1016/S0008-8846(03)00254-0
Aydin S, Baradan B (2014) Effect of activator type and content on properties of alkali-activated slag mortars. Compos B Eng 57:166–172. https://doi.org/10.1016/j.compositesb.2013.10.001
Puertas F, Fernández-Jiménez A (2003) Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem Concr Compos 25(3):287–292. https://doi.org/10.1016/S0958-9465(02)00059-8
Yang T, Yao X, Zhang Z, Wang H (2012) Mechanical property and structure of alkali-activated fly ash and slag blends. J Sustain Cem Based Mater 1(4):167–178. https://doi.org/10.1080/21650373.2012.752621
Aydin S, Baradan B (2012) Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater Des 35:374–383. https://doi.org/10.1016/j.matdes.2011.10.005
Acknowledgements
This paper is a scientific output of the research actions performed in the framework of the FWO-EOS project 30439691 ‘INTERdisciplinary multiscale Assessment of a new generation of Concrete with alkali-activated maTerials’ (INTERACT). The financial support by FWO-EOS is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
XD: Methodology, Conceptualization, Investigation, Writing—original draft; SA: Methodology, Conceptualization, Writing—review editing; MYY: Methodology, Validation, Writing – review editing; YS: Methodology, Writing—review editing; GDS: Funding acquisition, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, X., Aydin, S., Yardimci, M.Y. et al. Effect of temperature on the fresh and hardened state properties of alkali-activated slag/fly ash mixtures. Mater Struct 56, 107 (2023). https://doi.org/10.1617/s11527-023-02194-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-023-02194-w