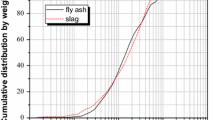
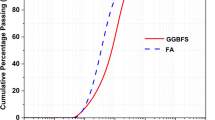
Abstract
This study focused on characterizing the carbonation behavior of 90-day aged alkali-activated fly ash/slag blended by MgO. Effects of MgO reactivity on the carbonation behavior of the synthesized binders were explored. A 0.3% CO2 concentration was adopted for an accelerated carbonation environment. The samples were characterized using compressive strength tests, X-ray diffraction, thermogravimetry, 27Al solid-state magic angle spinning nuclear magnetic resonance spectroscopy, and scanning electron microscopy with energy-dispersive spectroscopy. Notably, the compressive strengths of all the samples significantly increased after 28 days of carbonation. Moreover, aragonite was identified as the major carbonation product formed in all samples; nevertheless, its precipitation was scarcely affected by the degree of MgO reactivity. In particular, carbonation results in the decalcification of calcium silicate hydrate gels and the formation of layered double hydroxides. The results illustrate that the sample with relatively higher MgO reactivity exhibits improved carbonation durability.








Similar content being viewed by others
References
Provis JL (2018) Alkali-activated materials. Cem Concr Res 114:40–48. https://doi.org/10.1016/j.cemconres.2017.02.009
Zhang J, Shi C, Zhang Z, Ou Z (2017) Durability of alkali-activated materials in aggressive environments: a review on recent studies. Constr Build Mater 152:598–613. https://doi.org/10.1016/j.conbuildmat.2017.07.027
Peng L, Stewart MG (2016) Climate change and corrosion damage risks for reinforced concrete infrastructure in China. Struct Infrastruct Eng 12:499–516. https://doi.org/10.1080/15732479.2013.858270
Bernal SA, de Gutierrez RM, Provis JL, Rose V (2010) Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem Concr Res 40:898–907. https://doi.org/10.1016/j.cemconres.2010.02.003
Li N, Farzadnia N, Shi C (2017) Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem Concr Res 100:214–226. https://doi.org/10.1016/j.cemconres.2017.07.008
Johannesson B, Utgenannt P (2001) Microstructural changes caused by carbonation of cement mortar. Cem Concr Res 31:925–931. https://doi.org/10.1016/S0008-8846(01)00498-7
Steffens A, Dinkler D, Ahrens H (2002) Modeling carbonation for corrosion risk prediction of concrete structures. Cem Concr Res 32:935–941. https://doi.org/10.1016/S0008-8846(02)00728-7
Papadakis VG, Vayenas CG, Fardis MN (1991) Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem Eng Sci 46:1333–1338. https://doi.org/10.1016/0009-2509(91)85060-B
Bernal SA, Provis JL (2014) Durability of alkali-activated materials: Progress and perspectives. J Am Ceram Soc 97:997–1008. https://doi.org/10.1111/jace.12831
Provis JL, van Deventer JSJ (2014) Alkali activated materials: state-of-the-Art report. Springer, Netherlands
Bernal SA, Provis JL, Walkley B, San Nicolas R, Gehman JD, Brice DG, Kilcullen AR, Duxson P, Van Deventer JSJ (2013) Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem Concr Res 53:127–144. https://doi.org/10.1016/j.cemconres.2013.06.007
Bernal SA, San Nicolas R, Provis JL, Mejía De Gutiérrez R, Van Deventer JSJ (2014) Natural carbonation of aged alkali-activated slag concretes, Materials and Structures/Materiaux et. Constructions 47:693–707. https://doi.org/10.1617/s11527-013-0089-2
Palacios M, Puertas F (2006) Effect of carbonation on alkali-activated slag paste. J Am Ceram Soc 89:3211–3221. https://doi.org/10.1111/j.1551-2916.2006.01214.x
Sufian Badar M, Kupwade-Patil K, Bernal SA, Provis JL, Allouche EN (2014) Corrosion of steel bars induced by accelerated carbonation in low and high calcium fly ash geopolymer concretes. Constr Build Mater 61:79–89. https://doi.org/10.1016/j.conbuildmat.2014.03.015
Puertas F, Palacios M, Vázquez T (2006) Carbonation process of alkali-activated slag mortars. J Mater Sci 41:3071–3082. https://doi.org/10.1007/s10853-005-1821-2
Bernal SA, San Nicolas R, Myers RJ, De Mejía Gutiérrez R, Puertas F, Van Deventer JSJ, Provis JL (2014) MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem Concr Res 57:33–43. https://doi.org/10.1016/j.cemconres.2013.12.003
Mo L, Panesar DK (2012) Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem Concr Res 42:769–777. https://doi.org/10.1016/j.cemconres.2012.02.017
Vandeperre LJ, Al-Tabbaa A (2007) Accelerated carbonation of reactive MgO cements. Adv Cem Res 19:1–13
Wang Z, Park S, Khalid HR, Lee HK (2021) Hydration properties of alkali-activated fly ash/slag binders modified by MgO with different reactivity. J Build Eng. 44:103252. https://doi.org/10.1016/j.jobe.2021.103252
Bernal SA, Provis JL, Brice DG, Kilcullen A, Duxson P, Van Deventer JSJ (2012) Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: the role of pore solution chemistry. Cem Concr Res 42:1317–1326. https://doi.org/10.1016/j.cemconres.2012.07.002
Arbi K, Nedeljković M, Zuo Y, Ye G (2016) A review on the durability of Alkali-activated fly Ash/Slag systems: advances, issues, and perspectives. Ind Eng Chem Res 55:5439–5453. https://doi.org/10.1021/acs.iecr.6b00559
Bernal SA, Provis JL, Mejía de Gutiérrez R, van Deventer JSJ (2014) Accelerated carbonation testing of alkali-activated slag/metakaolin blended concretes: effect of exposure conditions. Mater Struct/Materiaux et Constr 48:653–669. https://doi.org/10.1617/s11527-014-0289-4
Galan I, Andrade C, Castellote M (2013) Natural and accelerated CO2 binding kinetics in cement paste at different relative humidities. Cem Concr Res 49:21–28. https://doi.org/10.1016/j.cemconres.2013.03.009
B. Lagerblad (2005), Carbon dioxide uptake during concrete life cycle - State of the art
Bakharev T, Sanjayan JG, Cheng YB (2001) Resistance of alkali-activated slag concrete to carbonation. Cem Concr Res 31:1277–1283. https://doi.org/10.1016/S0008-8846(01)00574-9
Houst YF (1996) The role of moisture in the carbonation of cementitious materials. Int J Restor Build Monum 2:49–66
Houst YF, Wittmann FH (1994) Influence of porosity and water content on the diffusivity of CO2 and O2 through hydrated cement paste. Cem Concr Res 24:1165
Li Z, Zhang W, Wang R, Chen F, Jia X, Cong P (2019) Effects of reactive MgO on the reaction process of geopolymer. Materials 12:1–10. https://doi.org/10.3390/ma12030526
Swamy RN, Bouikni A (1990) Some engineering properties of slag concrete as influenced by mix proportioning and curing. Mater J 87:210–220
Collins F, Sanjayan JG (2001) Microcracking and strength development of alkali activated slag concrete. Cem Concr Compos 23:345–352. https://doi.org/10.1016/S0958-9465(01)00003-8
Duran Atiş C, Bilim C, Çelik Ö, Karahan O (2009) Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr Build Mater 23:548–555. https://doi.org/10.1016/j.conbuildmat.2007.10.011
Zhang J, Shi C, Zhang Z (2019) Carbonation induced phase evolution in alkali-activated slag/fly ash cements: the effect of silicate modulus of activators. Constr Build Mater 223:566–582. https://doi.org/10.1016/j.conbuildmat.2019.07.024
Zhang Z, Chen S, Zhang Y (2019) Effect of hydrotalcite-like compounds with high specific surface area on mechanical properties and carbonation resistance of cementitious composites. Mater Res Express 6(11):115099. https://doi.org/10.1088/2053-1591/ab4b89
Bernal SA (2015) Effect of the activator dose on the compressive strength and accelerated carbonation resistance of alkali silicate-activated slag/metakaolin blended materials. Constr Build Mater 98:217–226. https://doi.org/10.1016/j.conbuildmat.2015.08.013
Shi Z, Shi C, Wan S, Li N, Zhang Z (2018) Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem Concr Res 113:55–64. https://doi.org/10.1016/j.cemconres.2018.07.005
De Leeuw NH, Parker SC (1998) Surface structure and morphology of calcium carbonate polymorphs calcite, aragonite, and vaterite: an atomistic approach. J Phys Chem B 102:2914–2922. https://doi.org/10.1021/jp973210f
Domingo C, Loste E, Gómez-Morales J, García-Carmona J, Fraile J (2006) Calcite precipitation by a high-pressure CO2 carbonation route. J Supercrit Fluids 36:202–215. https://doi.org/10.1016/j.supflu.2005.06.006
Stepkowska ET (2003) Calcite, vateite and aragonite forming on cement hydration from liquid and gaseous phase. J Therm Anal Calorim 73:247–269
Ye H, Huang L (2020) Degradation mechanisms of alkali-activated binders in sulfuric acid: the role of calcium and aluminum availability. Constr Build Mater 246:118477. https://doi.org/10.1016/j.conbuildmat.2020.118477
Kapeluszna E, Kotwica Ł, Różycka A, Gołek Ł (2017) Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr Build Mater 155:643–653. https://doi.org/10.1016/j.conbuildmat.2017.08.091
Mejía JM, Rodríguez E, Mejía De Gutiérrez R, Gallego N (2015) Preparation and characterization of a hybrid alkaline binder based on a fly ash with no commercial value. J Clean Prod 104:346–352. https://doi.org/10.1016/j.jclepro.2015.05.044
Theiss FL, Ayoko GA, Frost RL (2013) Thermogravimetric analysis of selected layered double hydroxides. J Therm Anal Calorim 112:649–657. https://doi.org/10.1007/s10973-012-2584-z
Park S, Park HM, Yoon HN, Seo J, Yang C-M, Provis JL, Yang B (2020) Hydration kinetics and products of MgO-activated blast furnace slag. Constr Build Mater 249:118700. https://doi.org/10.1016/j.conbuildmat.2020.118700
Yu P, Kirkpatrick R (1999) Thermal dehydation of tobermorite and jennite. Concr Sci Eng 1:185–191
Nonat A (2004) The structure and stoichiometry of C-S-H. Cem Concr Res 34:1521–1528. https://doi.org/10.1016/j.cemconres.2004.04.035
Parker LM, Milestone NB, Newman RH (1995) The use of hydrotalcite as an anion absorbent. Ind Eng Chem Res 34:1196–1202. https://doi.org/10.1021/ie00043a023
Bernard E, Dauzères A, Lothenbach B (2018) Magnesium and calcium silicate hydrates, part II: Mg-exchange at the interface “low-pH” cement and magnesium environment studied in a C-S-H and M-S-H model system. Appl Geochem 89:210–218. https://doi.org/10.1016/j.apgeochem.2017.12.006
Bernard E, Lothenbach B, Cau-Dit-Coumes C, Chlique C, Dauzères A, Pochard I (2018) Magnesium and calcium silicate hydrates, Part I: Investigation of the possible magnesium incorporation in calcium silicate hydrate (C-S-H) and of the calcium in magnesium silicate hydrate (M-S-H). Appl Geochem 89:229–242. https://doi.org/10.1016/j.apgeochem.2017.12.005
Provis JL (2014) Geopolymers and other alkali activated materials: Why, how, and what? Mater Struct/Materiaux et Constr 47:11–25. https://doi.org/10.1617/s11527-013-0211-5
Dung NT, Hay R, Lesimple A, Celik K, Unluer C (2021) Influence of CO2 concentration on the performance of MgO cement mixes. Cement Concr Compos 115:103826. https://doi.org/10.1016/j.cemconcomp.2020.103826
Park SM, Jang JG, Lee HK (2018) Unlocking the role of MgO in the carbonation of alkali-activated slag cement. Inorg Chem Front 5:1661–1670. https://doi.org/10.1039/c7qi00754j
Mackenzie KJD, Meinhold RH, Sherriff BL, Xu Z (1993) 27Al and25Mg solid-state magic-angle spinning nuclear magnetic resonance study of hydrotalcite and its thermal decomposition sequence. J Mater Chem 3:1263–1269. https://doi.org/10.1039/JM9930301263
Rowles MR, Hanna JV, Pike KJ, Smith ME, O’Connor BH (2007) 29Si, 27Al, 1H and 23Na MAS NMR study of the bonding character in aluminosilicate inorganic polymers. Appl Magn Reson 32:663–689. https://doi.org/10.1007/s00723-007-0043-y
Fernández-Jiménez A, Palomo A (2005) Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater 86:207–214. https://doi.org/10.1016/j.micromeso.2005.05.057
Singh PS, Trigg M, Burgar I, Bastow T (2005) Geopolymer formation processes at room temperature studied by 29Si and 27Al MAS-NMR. Mater Sci Eng, A 396:392–402. https://doi.org/10.1016/j.msea.2005.02.002
Bernard E, Lothenbach B, Cau-Dit-Coumes C, Pochard I, Rentsch D (2020) Aluminum incorporation into magnesium silicate hydrate (M-S-H). Cem Concr Res 128:105931. https://doi.org/10.1016/j.cemconres.2019.105931
Longhi MA, Walkley B, Rodríguez ED, Kirchheim AP, Zhang Z, Wang H (2019) New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos Part B: Eng. 176:107172. https://doi.org/10.1016/j.compositesb.2019.107172
Walkley B, San Nicolas R, Sani MA, Gehman JD, Van Deventer JSJ, Provis JL (2016) Phase evolution of Na2O-Al2O3-SiO2-H2O gels in synthetic aluminosilicate binders. Dalton Trans 45:5521–5535. https://doi.org/10.1039/c5dt04878h
Duxson P, Lukey GC, Separovic F, Van Deventer JSJ (2005) Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind Eng Chem Res 44:832–839. https://doi.org/10.1021/ie0494216
Park SM, Jang JG, Lee NK, Lee HK (2016) Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem Concr Res 89:72–79. https://doi.org/10.1016/j.cemconres.2016.08.004
Ismail I, Bernal SA, Provis JL, San Nicolas R, Hamdan S, Van Deventer JSJ (2014) Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem Concr Compos 45:125–135. https://doi.org/10.1016/j.cemconcomp.2013.09.006
Walkley B, San Nicolas R, Sani MA, Bernal SA, van Deventer JSJ, Provis JL (2017) Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem Concr Res 99:155–171. https://doi.org/10.1016/j.cemconres.2017.05.006
Fasihnikoutalab MH, Asadi A, Unluer C, Huat BK, Ball RJ, Pourakbar S (2017) Utilization of Alkali-activated olivine in soil stabilization and the effect of carbonation on unconfined compressive strength and microstructure. J Mater Civ Eng 29:06017002. https://doi.org/10.1061/(asce)mt.1943-5533.0001833
Sonat C, Unluer C (2019) Development of magnesium-silicate-hydrate (M-S-H) cement with rice husk ash. J Clean Prod 211:787–803. https://doi.org/10.1016/j.jclepro.2018.11.246
** F, Al-Tabbaa A (2015) Strength and drying shrinkage of slag paste activated by sodium carbonate and reactive MgO. Constr Build Mater 81:58–65. https://doi.org/10.1016/j.conbuildmat.2015.01.082
** F, Gu K, Al-Tabbaa A (2014) Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr Build Mater 51:395–404. https://doi.org/10.1016/j.conbuildmat.2013.10.081
Villagrán-Zaccardi YA, Egüez-Alava H, De Buysser K, Gruyaert E, De Belie N (2017) Calibrated quantitative thermogravimetric analysis for the determination of portlandite and calcite content in hydrated cementitious systems. Mater Struct/Materiaux et Constr. https://doi.org/10.1617/s11527-017-1046-2
Dauzeres A, Achiedo G, Nied D, Bernard E, Alahrache S, Lothenbach B (2016) Magnesium perturbation in low-pH concretes placed in clayey environment—solid characterizations and modeling. Cem Concr Res 79:137–150. https://doi.org/10.1016/j.cemconres.2015.09.002
Lothenbach B, Nied D, L’Hôpital E, Achiedo G, Dauzères A (2015) Magnesium and calcium silicate hydrates. Cem Concr Res 77:60–68. https://doi.org/10.1016/j.cemconres.2015.06.007
Ul Haq E, Padmanabhan SK, Licciulli A (2014) In-situ carbonation of alkali activated fly ash geopolymer. Constr Build Mater 66:781–786. https://doi.org/10.1016/j.conbuildmat.2014.06.012
Zhang Y, Li Y, Xu Y, Sang S, ** S (2017) Enhanced formation of magnesium silica hydrates (M-S-H) using sodium metasilicate and caustic magnesia in magnesia castables. Ceram Int 43:9110–9116. https://doi.org/10.1016/j.ceramint.2017.04.058
Zhang Y, Li Y, Dai Y (2018) Formation of magnesium silicate hydrate in the Mg(OH)2-SiO2 suspensions and its influence on the properties of magnesia castables. Ceram Int 44:21365–21373. https://doi.org/10.1016/j.ceramint.2018.08.190
Zhang T, Zou J, Wang B, Wu Z, Jia Y, Cheeseman CR (2018) Characterization of Magnesium silicate hydrate (MSH) gel formed by reacting MgO and silica fume. Materials 11:1–15. https://doi.org/10.3390/ma11060909
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF), South Korea, grant funded by the Korean government (Ministry of Science and ICT) (No. 2021R1A2C3006382). NMR data were acquired using a 400MHz Solid State NMR spectrometer (AVANCE III HD, Bruker, Germany) at the KBSI Western Seoul Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Park, S., Khalid, H.R. et al. Carbonation behavior of aged alkali-activated fly ash/slag binder modified by MgO with different reactivities. Mater Struct 57, 119 (2024). https://doi.org/10.1617/s11527-024-02397-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-024-02397-9