Abstract
Rapid migration of mesenchymal stem cells (MSCs) on device surfaces could support in vivo tissue integration and might facilitate in vitro organoid formation. Here, polydopamine (PDA) is explored as a biofunctional coating to effectively promote MSC motility. It is hypothesized that PDA stimulates fibronectin deposition and in this way enhances integrin-mediated migration capability. The random and directional cell migration was investigated by time-lapse microscopy and gap closure assay respectively, and analysed with softwares as computational tools. A higher amount of deposited fibronectin was observed on PDA substrate, compared to the non-coated substrate. The integrin β1 activation and focal adhesion kinase (FAK) phosphorylation at Y397 were enhanced on PDA substrate, but the F-actin cytoskeleton was not altered, suggesting MSC migration on PDA was regulated by integrin initiated FAK signalling. This study strengthens the biofunctionality of PDA coating for regulating stem cells and offering a way of facilitating tissue integration of devices.
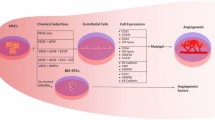
Graphic abstract
Polydopamine-coated substrate induces increased fibronectin deposition of mesenchymal stem cells, and promotes cell migration via integrin-initiated FAK signaling, compared to non-coated polystyrene-based standard tissue culture surface. In this way, multifunctional PDA coating could support in vivo tissue integration on implant surface and promote in vitro organoid formation.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A multifunctional layer created at the interface of stem cells and biomaterials, for example through the coating and modification of cell culture material or implant surface, would play a crucial role to regulate and control the behavior and function of stem cells and thereby to enhance the beneficial effect in biomedical applications [1, 2]. The implant surface, as part of the microenvironment presented to the cells, should facilitate the cell adhesion, proliferation, migration and differentiation to support tissue integration and reduce the foreign body reaction [3, 4]. In addition, precise control of stem cells and tissue architecture is of great importance for formation of organoids, which hold great potential as a model system for biology study and disease treatment [5,6,7].
Inspired by the natural phenomena of mussel-adhesion, polydopamine (PDA) was first introduced in 2007 [8], and to date has become one of the most prominent biomimetic materials. PDA coatings were proven to be able to promote adhesion, proliferation, and differentiation of stem cells, and hence have been used in tissue engineering to modify the surface of metal-, polymer- and carbon-based implants [9, 10]. The large number of catechol and amine based moieties in PDA enable the strong binding of PDA to a wide range of surfaces and meanwhile allow the adhesion of functional biomolecules through Schiff base reaction and Michael addition to facilitate cell attachment [8, 11,12,13]. For example, the endothelial cells were found to neither attach nor proliferate on the poly(l-lysine) (PLL) coated substrate which was widely used to assist cell attachment [14, 15], but well adhere to the PDA substrate [16]. We explore PDA as a multifunctional substrate coating, which fulfils the design principles of substratum for adherent cells [17]. In our recent work two biofunctions of PDA coatings were unravelled: preventing mesenchymal stem cells (MSCs) from replicative cellular senescence and strongly promoting MSC proliferation [18]. In addition, PDA coatings enhanced the adhesion, stability and differentiation of MSCs as reported in [13, 19, 20].
Given that the therapeutic efficacy of MSCs for implant integration in tissue highly relies on their migration and engraftment into the tissue at the implant interface [21, 22], we hypothesized here that a PDA coating can also promote MSC migration and in this way exhibit an additional biofunction. In this study, the PDA coating was applied to a polystyrene based standard tissue culture plate (TCP) via polymerization of dopamine solution in alkaline buffer. MSC migration on PDA substrate was examined with time-lapse microscopy and gap closure assay. The softwares were applied as computational tools to precisely trace and analyse the cell migration. The mechanism that PDA coated substrate promotes MSC migration was investigated, focusing on fibronectin deposition, integrin activation, cytoskeleton organization and focal adhesion kinase (FAK) phosphorylation.
Experimental details
The multifunctional PDA coatings were prepared on polystyrene based standard tissue culture plates (TCPs) via polymerization of dopamine solution in alkaline buffer. The random and directional migration of human MSCs was measured using time-lapse microscopy and gap closure assay respectively. The data were analysed with ImageJ and Image-Pro Plus softwares. The samples were stained to identify the fibronectin, active integrin, F-actin and pFAK (Y397). The protein levels were quantified by measuring the fluorescence intensity and ELISA assay. See supporting information for detail of the methods.
Discussion
The random MSC movement on different substrates was first examined using a time-lapse microscope. Given the shape change of the cells during migration, the cell nuclei were stained with the live cell fluorescent dye (Hoechst 33342) to record the migration trace (Fig. 1a). The PLL substrate was included here as a reference material, which has been prove to largely increase MSC migration [23]. Compared to the control surface (non-coated TCP), the PLL and PDA-0.1 substrates significantly increased the migration velocity of MSCs (Fig. 1b). A longer straight migration distance was observed in the cells on PLL substrate (Fig. 1c). The gap closure on different surfaces was performed to assess the collective cell migration (Fig. 1d). Compared to the random migration assay, this approach measured the directional migration, since the cells would form lamellipodia protrusion and move towards the wound gap. The gap images at indicated time points were processed with Image-Pro Plus software to minimize the influence of background and contrast, and to precisely measure the wound gap area. Our results indicated that the percentage of gap closure was significantly increased on the PLL and PDA substrates, as compared to the non-coated TCP (Fig. 1e).
Migration of MSCs on TCP and coated substrates after 3 days of culture. Time-lapse microscopy was applied to trace the stained cell nuclei for 8 h, and the random cell migration was analyzed using ImageJ software to generate the migration trajectories (a) and to quantify the moving velocity (b) and straight moving distance (c) (n ≥ 23; mean ± standard error of the mean). The directional migration was examined by gap closure assay. Representative images showed the gaps immediately after insert removal (0 h) and after 24 h (d, bar = 200 μm). The percentage of gap closure was quantified by Image-Pro Plus software (E; n = 8). The images were processed to minimize the influence of background and contrast, and for each image an area with a length of 1.0 mm along the gap was measured. *p < 0.05
The complex process of cell migration is orchestrated by cell adhesion to extracellular matrix (ECM), integrin activation, intracellular signalling cascade, actomyosin organization and cytoskeleton dynamics. The surface properties of PDA layer, such as topography, hydrophilicity and surface charge, play a combinational role to modulate protein adsorption and cell interaction at the cell-material interface. In our previous work, we have characterized the surface nano-structure, hydrophilicity and protein adsorption capacity of PDA coated substrates [18]. Compared to the non-coated TCP, the PDA coating led to increased nano-roughness. A higher amount and smaller nano-aggregates were found on PDA-0.1 than on PDA-0.5 substrate. The effect that the nano-structures on material surface could stimulate cell migration as described in [24, 25], is in consistence with our findings. The PDA coated substrates showed a higher hydrophilicity than non-coated TCP, which could favor the cell attachment [26]. In addition, PDA coating significantly enhanced the protein adsorption capacity of the substrate. The surface charge of the material is crucial to influence the surface chemistry, protein adsorption, as well as the interaction with cells. Here, the PDA layer would show a negative surface charge in cell culture medium (pH 7.4) [27]. Given the potential influence of these surface properties on cells, we first examined the fibronectin deposition of MSCs on the substrates in order to understand the mechanism through which the PDA coating increases the MSC migration. It has been reported that cell adhesion to fibronectin through integrin α5β1 could activate the PDGFR-β signalling and trigger the migration of MSCs [28]. Here, the level of fibronectin deposition on PDA-0.1 substrate was significantly higher than on non-coated TCP control (Fig. 2a, d). However, the intracellular fibronectin was at a similar level for all groups (Fig. 2e).
Fibronectin deposition, integrin activation and FAK phosphorylation of MSCs cultured on different substrates for 3 days. Representative staining images showed the fibronectin (green in a), active integrin β1 (green in b), pFAK (Y397) (red in c) and cell nuclei (blue) (bar = 50 μm). d Fluorescence intensity of stained fibronectin was quantified using ImageJ software and normalized by cell number (n ≥ 4). The value in TCP group was set 1. e Fibronectin amount in cell lysis was measured using ELISA and was normalized with the total protein amount (n = 4). f Fluorescence intensity of pFAK (Y397) was quantified using ImageJ software and normalized by cell number (n = 4). The quantity of pFAK (Y397) (g) and total FAK (h) in cell lysis was determined using ELISA, and was normalized with the total protein amount (n = 4). *p < 0.05
Integrins are transmembrane receptors that bind to both extracellular and intracellular ligands and regulate the transmission of mechanical and biochemical signals [29]. The dynamic interaction of integrins with the ECM and the actin cytoskeleton play a crucial role in cell migration [30]. Upon integrin activation, a rapid and reversible conformational change in the extracellular domain resulted in the increased integrin affinity to extracellular ligand [31, 32]. Fibronectin could be recognized by integrin α5β1 through the arg–gly–asp (RGD) cell-binding sequence [33], and the fibronectin-integrin α5β1 interaction could in turn regulate the fibronectin matrix assembly [34]. Given the enhanced fibronectin deposition on PDA-0.1 substrate, we stained the cells to assess the level of integrin β1 activation. A slightly increase of the fluorescence intensity of active integrin β1 was observed on PLL and PDA-0.1 substrates, as compared to the non-coated TCP (Fig. 2b), suggesting the enhanced integrin engagement on these substrates.
The cell migration is driven by the dynamic assembly and disassembly of actin and associated proteins. Actin polymerization regulates extension of the lamellipodia and filopodia protrusions at the leading edge of a migrating cell. In addition, actin connects with integrins and interacts with myosin to generate the intracellular contraction forces to trail the cell body [35, 36]. Here, we did not observe the marked difference of F-actin cytoskeleton structure and organization on different surfaces. The cells showed the strong and well-oriented F-actin stress fibers in all tested groups (Fig. S1).
Focal adhesion kinase (FAK), as a cytoplasmic tyrosine kinase, plays an important role in integrin-mediated signal transduction and cell migration through regulating cytoskeleton dynamics and focal adhesion turnover [22, 37, 38]. Upon cell adhesion, FAK could be recruited to focal adhesions, and the autophosphorylation of FAK at Y397 would be initiated by the binding of integrins to ECM and their clustering. The kinase activity of FAK can be fully activated via its phosphorylation at Y397 [39]. The regulatory role of FAK in MSC migration has been reported in different studies [40,41,42,43,44,45]. Inhibition of FAK resulted in the suppressed cellular motility of MSCs [46, 47]. Here, compared to non-coated TCP, FAK phosphorylation at Y397 was significantly upregulated on PLL and PDA-0.1 substrates, as shown by immunostaining images (Fig. 2c, f) and quantification assay (Fig. 2g). Notably, the level of FAK phosphorylation in PDA-0.5 group was lower than in PDA-0.1 group, which might be attributed to the influence of surface topography. According to our previous study, PDA-0.5 displayed a lower amount but larger PDA aggregates on the surface than PDA-0.1 [18], which might induce different cellular response of MSCs. The total FAK was kept at a similar level in all groups (Fig. 2h). Taken together, these results suggested that PDA promoted MSC migration through the activation of integrin and FAK signalling.
Conclusions
In this study, we investigate the MSC migration on PDA coated TCP substrate and elucidated the cellular mechanism. Compared to the non-coated TCP control, PDA substrate promoted both random and directional migration of MSCs. The cells on PDA substrate deposited a higher amount of fibronectin, and showed the increased level of integrin β1 activation and FAK phosphorylation, suggesting the predominant role of integrin initiated FAK signalling in MSC migration on PDA. The multifunctionality of PDA coating highlights its application in cell culture and implant surface modification for tissue integration. Precise control of stem cells with PDA substrates shows great potential for promoting organoid formation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
R.H. Fu, Y.C. Wang, S.P. Liu, C.M. Huang, Y.H. Kang, C.H. Tsai, W.C. Shyu, S.Z. Lin, Cell Transpl. 20(1), 37 (2011)
N.R. Richbourg, N.A. Peppas, V.I. Sikavitsas, J. Tissue Eng. Regen M 13(8), 1275 (2019)
C.X. Yue, H.C. van der Mei, R. Kuijer, H.J. Busscher, E.T.J. Rochford, J. Biomed. Mater. Res. A 103(11), 3590 (2015)
L.Y. Liu, G. Chen, T. Chao, B.D. Ratner, E.H. Sage, S.Y. Jiang, J. Biomater. Sci. Polym. E 19(6), 821 (2008)
X.L. Yin, B.E. Mead, H. Safaee, R. Langer, J.M. Karp, O. Levy, Cell Stem Cell 18(1), 25 (2016)
M. Hofer, M.P. Lutolf, Nat. Rev. Mater. (2021). https://doi.org/10.1038/s41578-021-00279-y
J. Kim, B.K. Koo, J.A. Knoblich, Nat. Rev. Mol. Cell Bio. 21(10), 571 (2020)
H. Lee, S.M. Dellatore, W.M. Miller, P.B. Messersmith, Science 318(5849), 426 (2007)
N. Kaushik, L.N. Nguyen, J.H. Kim, E.H. Choi, N.K. Kaushik, Int. J. Mol. Sci. 21(18), 6544 (2020)
J. Yan, R.Y. Wu, S.S. Liao, M. Jiang, Y. Qian, Front. Bioeng. Biotechnol. 8, 590998 (2020)
H.A. Lee, Y.F. Ma, F. Zhou, S. Hong, H. Lee, Accounts Chem. Res. 52(3), 704 (2019)
K. Patel, N. Singh, J. Yadav, J.M. Nayak, S.K. Sahoo, J. Lata, D. Chand, S. Kumar, R. Kumar, Phys. Chem. Chem. Phys. 20(8), 5744 (2018)
Y.J. Chuah, Y.T. Koh, K. Lim, N.V. Menon, Y. Wu, Y. Kang, Sci. Rep. Uk 5, 18162 (2015)
Y. Choi, A.K. Yagati, S. Cho, J. Nanosci. Nanotechnol. 15(10), 7881 (2015)
D.D. Zhang, Y.M. Zhang, L. Zheng, Y.Z. Zhan, L.C. He, Biosens. Bioelectron. 42, 112 (2013)
Y. Zhang, M.E. Lynge, B.M. Teo, R. Ogaki, B. Stadler, Biomater. Sci. Uk 3(8), 1188 (2015)
N. Scharnagl, S. Lee, B. Hiebl, A. Sisson, A. Lendlein, J. Mater. Chem. 20(40), 8789 (2010)
Z. Deng, W. Wang, X. Xu, Y. Nie, Y. Liu, O.E.C. Gould, N. Ma, A. Lendlein, ACS Appl. Mater. Interfaces 13(9), 10748 (2021)
D. Sharma, W.K. Jia, F. Long, S. Pati, Q.H. Chen, Y.B. Qyang, B. Lee, C.K. Choi, F. Zhao, Bioact. Mater. 4, 142 (2019)
D.J. Lee, Y.T. Lee, R. Zou, R. Daniel, C.C. Ko, Sci. Rep. Uk 7, 12984 (2017)
J. Leibacher, R. Henschler, Stem Cell Res. Ther. (2016). https://doi.org/10.1186/s13287-015-0271-2
X.R. Fu, G. Liu, A. Halim, Y. Ju, Q. Luo, G.B. Song, Cells Basel 8(8), 784 (2019)
C. Somaiah, A. Kumar, D. Mawrie, A. Sharma, S.D. Patil, J. Bhattacharyya, R. Swaminathan, B.G. Jaganathan, PLoS ONE 10(12), e0145068 (2015)
W.M.A. Maximiano, V.M. Mazucato, P.T. de Oliveira, M.C. Jamur, C. Oliver, J. Biomed. Mater. Res. A 105(8), 2150 (2017)
E.G. Long, M. Buluk, M.B. Gallagher, J.M. Schneider, J.L. Brown, Bioact. Mater. 4, 249 (2019)
Y. Tamada, Y. Ikada, Polymer 34(10), 2208 (1993)
Y.L. Liu, K.L. Ai, L.H. Lu, Chem. Rev. 114(9), 5057 (2014)
J. Veevers-Lowe, S.G. Ball, A. Shuttleworth, C.M. Kielty, J. Cell Sci. 124(8), 1288 (2011)
S.J. Shattil, C. Kim, M.H. Ginsberg, Nat. Rev. Mol. Cell Bio. 11(4), 288 (2010)
A. Huttenlocher, A.R. Horwitz, CSH Perspect. Biol. 3(9), a005074 (2011)
D.A. Calderwood, I.D. Campbell, D.R. Critchley, Nat. Rev. Mol. Cell Bio. 14(8), 503 (2013)
D.A. Calderwood, J. Cell Sci. 117(5), 657 (2004)
F. Schaffner, A.M. Ray, M. Dontenwill, Cancers (Basel) 5(1), 27 (2013)
J.L. Sechler, S.A. Corbett, J.E. Schwarzbauer, Mol. Biol. Cell 8(12), 2563 (1997)
M. Vicente-Manzanares, C.K. Choi, A.R. Horwitz, J. Cell Sci. 122(2), 199 (2009)
M. Schaks, G. Giannone, K. Rottner, Essays Biochem. 63(5), 483 (2019)
X. Zhao, J.L. Guan, Adv. Drug Deliver Rev. 63(8), 610 (2011)
Z.D. Li, W.W. Wang, X. Xu, K. Kratz, J. Zou, L. Lysyakova, M. Heuchel, A. Kurtz, M. Gossen, N. Ma, A. Lendlein, J. Mater. Chem. B 5(35), 7415 (2017)
C.T. Mierke, Phys. Biol. (2013). https://doi.org/10.1088/1478
F.B. Meng, Y.F. Rui, L.L. Xu, C. Wan, X.H. Jiang, G. Li, Stem Cells Dev. 23(1), 66 (2014)
H. Gao, W. Priebe, J. Glod, D. Banerjee, Stem Cells 27(4), 857 (2009)
A.S. Zhu, N.X. Kang, L.H. He, X.Y. Li, X.J. Xu, H.X. Zhang, J. Cell Biochem. 117(6), 1370 (2016)
X.M. Lu, J.B. Han, X.P. Xu, J.Y. Xu, L. Liu, Y.Z. Huang, Y. Yang, H.B. Qiu, Stem Cells Int. (2017). https://doi.org/10.1155/2017/8178643
C.Y. Zou, Q. Luo, J. Qin, Y.S. Shi, L. Yang, B.F. Ju, G.B. Song, Cell Biochem. Biophys. 65(3), 455 (2013)
Z.D. Li, X. Xu, W.W. Wang, K. Kratz, X.L. Sun, J. Zou, Z.J. Deng, F. Jung, M. Gossen, N. Ma, A. Lendlein, Clin. Hemorheol. Micro. 67(3–4), 267 (2017)
B.D. Riehl, J.S. Lee, L. Ha, J.Y. Lim, J. R. Soc. Interface 12(104), 20141351 (2015)
B.Y. Zhang, Q. Luo, Z. Chen, J.H. Sun, B.Y. Xu, Y. Ju, G.B. Song, Stem Cell Res. 14(2), 155 (2015)
Acknowledgments
This work was financially supported by the Helmholtz Association of German Research Centers through program-oriented funding, and I2B Funds (Project: high-resolution imaging and computational analysis to study the dynamics of stem cell-biomaterial interaction) as well as by the Federal Ministry of Education and Research, Germany, through the Program Health Research (Grant No. 13GW0098).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Nan Ma was an editor of this journal during the review and decision stage. For the MRS Advances policy on review and publication of manuscripts authored by editors, please refer to mrs.org/editor-manuscripts.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, Z., Wang, W., Xu, X. et al. Polydopamine-based biofunctional substrate coating promotes mesenchymal stem cell migration. MRS Advances 6, 739–744 (2021). https://doi.org/10.1557/s43580-021-00091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-021-00091-4