Abstract
A binder-free porous NiCu(CO3)(OH)2 composite was grown on a polyacrylonitrile (PAN) nanofiber substrate using a hydrothermal method. PAN nanofibers were fabricated by the electrospinning method, thus producing a substrate with a nano-sized diameter and high specific surface area. The composite NiCu(CO3)(OH)2 nanowires on PAN nanofibers provided the large specific surface area required for the redox reaction. Transition metal-based nanowires and nano-sized PAN substrates indicate a synergistic effect in electrochemical performance. The NiCu(CO3)(OH)2 on PAN composite showed a remarkable maximum specific capacity of 870 mAh g−1 at a current density of 3 A g−1, which indicates that it can be a suitable electrode material. In addition, an asymmetric supercapacitor with NiCu(CO3)(OH)2 on PAN composite as the cathode and graphene as the anode showed an ultra-high energy density of 89.2 W h kg−1 at a power density of 835 W kg−1 and a capacitance retention of 90.1% after 5000 cycles.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, energy consumption has increased worldwide as human well-being has become increasingly reliant on digital tools, and consequently, the need for high-performance energy storage systems has become prominent [1, 2]. The application of decentralized energy resources and storage technologies in the field of portable, flexible, and smart electronics has significant challenges that need to be addressed in the global context of energy transition [3, 4]. In this regard, flexible energy devices such as batteries and supercapacitors are key technologies for powering body-worn consumer electronics, sensors, or low-energy bioelectronics (e.g., on-skin diagnostics). Similarly, supercapacitors (SCs) that can fit into various device structures and installation spaces are key enablers for buffering excess energy. SCs have gained increasing attention owing to their fast charge and discharge rates, high power density, long cycle life, and high reliability [5,6,7]. However, endowing conventional SCs with good flexibility as well as high energy density is challenging because both charge collectors (substrates) and electrode materials can experience fracture and delamination during flexing. These supercapacitors can be classified into two types: electrical double-layer capacitors (EDLCs), which usually use carbon active materials, and faradaic capacitors, which use redox-active materials [8]. EDLC devices store electrochemical energy via electrostatic accumulation of charges in the electric double layer and currently dominate the SC market because of their ability to accumulate large amounts of charge [9]. However, their relatively low specific capacitance is inhibitive for the increasing requirements of SCs with higher electrochemical performance, which also restricts their potential large-scale applications [10, 11]. Recently, researchers have focused on exploring faradaic capacitors owing to their high specific capacitance, which is induced by fast reversible redox reactions [12]. Faradaic capacitors generally use a metal oxide/hydroxide as the cathode for efficient redox reaction on the electrode surface, which requires an electrode material with a large specific surface area and good electrochemical activity. Transition metal oxides and hydroxides [13, 14], such as NiO [15], Ni(OH)2 [16], Co3O4 [17], MnO2 [18], and RuO2 [19], and their binary composites have been considered as optimized materials for SCs. The reversible redox reaction in faradaic capacitors occurs on the surface of the electrode, and because the electrical energy is stored on the electrode surface, the storage capacity of faradaic capacitors is significantly influenced by electrode material properties. For this reason, binary NiCu-oxide/hydroxide compounds, which exhibit rich redox reactions due to their multiple oxidation states, have been widely used as faradaic capacitors electrodes. For example, Zhenyuan et al. fabricated NiO/Co3O4 ultrathin nanosheets that have a specific capacitance of 1775 F g−1 at 1 A g−1 [20]. Jun et al. fabricated Cu-metal organic frameworks (MOF) electrodes, which showed a specific capacitance of 318 F g−1 at 1 A g−1 in a KOH electrolyte [21]. Heba et al. prepared Ni–Cu binary phosphides, which exhibited a specific capacitance of 1573 F g−1 at 1 A g [22]. However, oxide and hydroxide composites normally suffer from gradually decreasing stability after several charging and discharging cycles [
Electrochemical properties
Figure 5a presents the redox curves of the electrodes with a three-electrode cell, which shows the cyclic voltammetry (CV) curves of the PAN@NiCu(CO3)(OH)2 composite in the potential window of 0.15–0.6 V at scan rates from 5 to 50 mV−1 in a 1 M KOH electrolyte. The oxidation and reduction peaks in the CV curves shifted toward more positive and negative potentials, respectively, as the scan speed increased because the internal diffusion resistance increased within the electrode surface. The typical CV peaks of the PAN@NiCu(CO3)(OH)2 electrodes showed reduction peak at ~ 0.46 V, and an oxidation peak at ~ 0.34 V, respectively, at 10 mV s−1. As the scan rate increased, the redox peak of the PAN@NiCu(CO3)(OH)2 electrode shifted to an extent, indicating that there is the internal resistance of the PAN@NiCu(CO3)(OH)2 electrode. And the CV of PAN@NiCu(CO3)(OH)2 has a large internal area, which is proportional to its capacity value. This result indicates that the PAN@NiCu(CO3)(OH)2 electrode has a great electrochemical value. This electrode exhibits symmetric redox peak property, indicating good performance for redox reactions and largely porous morphology. The CV curves obtained for the PAN@NiCu(CO3)(OH)2 nanowire electrode were standard for faradaic capacitors. The faradaic redox reactions of the NiCu(CO3)(OH)2 composite occurred according to Eqs. (1–3) [44, 45]:
According to these equations, the redox reactions involve OH− from the electrolyte in the optimized NiCu(CO3)(OH)2 composite. Figure 5b shows the galvanostatic charge–discharge (GCD) measurements of the PAN@NiCu(CO3)(OH)2 composite over a potential range of 0–0.45 V. The GCD curves showed a nonlinear slope and triangular symmetry owing to the occurrence of quasi-reversible redox reactions at the electrolyte/electrode interface. As shown in Fig. 5b, the PAN@NiCu(CO3)(OH)2 composite had a long discharge time, indicating that it has high electrochemical performance, which is consistent with the trend observed in the CV analysis. The specific capacities of the previously tested Ni-foam@NiCu(CO3)(OH)2 and PAN@NiCu(CO3)(OH)2 composites were calculated using the following Eq. (4) [46]:
where QD (mAh g−1) is the specific capacity, I (mA) is the discharging current, Δt (s) is the discharging time that is measured from 0.0 to 0.45 V, and m (g) is the designated mass of the active material. Using this equation, it was found that the PAN@NiCu(CO3)(OH)2 nanowire composite possessed high electrochemical values. As shown in Fig. 5c, the PAN@NiCu(CO3)(OH)2 electrode exhibited specific capacities of 870, 936, 1025, 960, 899, and 808 mAh g−1, while the specific capacities of the Ni-foam@NiCu(CO3)(OH)2 electrode were calculated to be 759, 632, 496, 424, 364, and 348 mAh g−1 at current densities of 3, 4, 5, 8, 10, and 15 A g−1, respectively. This was attributed to the positive effect of the increased surface area of the PAN@NiCu(CO3)(OH)2 electrode structure. As a result, PAN@NiCu(CO3)(OH)2 had higher specific capacitance values than the NiCu(CO3)(OH)2 composite on the Ni-foam substrate, which indicates the synergetic effect of the PAN nanofibers and NiCu(CO3)(OH)2 nanowire electrode. The cycling properties were also measured to investigate the stability of the electrodes. Long-term electrical retention is an important factor for electrodes in supercapacitor applications and industrialization [47]. As shown in Fig. 5d, after satisfactory cycles, the Ni-foam@NiCu(CO3)(OH)2 and PAN@NiCu(CO3)(OH)2 electrodes exhibited retentions of 88.2% and 84.1%, respectively, after 5000 cycles. The stability of the electrodes was evaluated using the discharging time at a constant current density of 10 A g−1. These results suggest that the Ni-foam@NiCu(CO3)(OH)2 electrode exhibited better cycling stability than the PAN@NiCu(CO3)(OH)2 electrode. The synergistic effects of the PAN substrate and NiCu(CO3)(OH)2 composite contributed to enhanced electrochemical performance for a specific capacitance; however, this electrode possessed a lower retention value owing to its weak polymer properties. Nevertheless, these results indicate better electrochemical properties than those previously reported for NiCu-based electrodes (Table 1). As a result, although the electrode was damaged and the capacity decreased with the increasing number of cycles, it had a high retention value because of the positive influence of the transition metal composites.
To further investigate the viability of the electrodes in energy storage devices, an ASC was fabricated using Ni-foam@NiCu(CO3)(OH)2 and PAN@NiCu(CO3)(OH)2 composites as the cathode and graphene as the anode. A cellulose separator paper and 1 M KOH electrolyte were used for the ASC device. A schematic diagram of the cathode and anode ASC electrodes is shown in Scheme 2. The electrochemical performance of the graphene electrode was measured in the potential range of − 1 to 0 V, as shown in Fig. 6. The CV curves of graphene showed typical rectangular shapes, which represent the ideal faradaic capacitor (Fig. 6a). The GCD curves exhibited linear and symmetric shapes, as shown in Fig. 6b. The specific capacity (Fig. 6c) of the graphene electrode was 122 mAh g−1 at 2 A g−1 and 60 mAh g−1 at 15 A g−1, respectively. EIS analyses were performed to investigate the conductivity behavior and charge-transfer kinetics of the graphene electrode. In the high-frequency region, the graphene electrode showed low Rs and Rct values of 0.6 Ω and 0.82 Ω, respectively (inset of Fig. 6d). The slope of the linear section of the graphene electrode has a higher incline in the low-frequency region, which represents a low Zw value, as shown in Fig. 6d. These results confirmed that the graphene electrode could be a suitable negative electrode for ASCs. Figure 7 shows the CV curves of the assembled ASC, which demonstrate electrical double-layer capacitor properties behavior at different scan rates in the voltage range of 0.0–1.5 V. This phenomenon indicates that PAN@NiCu(CO3)(OH)2 electrode//graphene ASCs were significantly influenced by graphene, which is a negative electrode material. As shown in Fig. 7a, the PAN@NiCu(CO3)(OH)2 composite shows excellent rectangular CV curves, which represent the ideal behavior of SCs. and this ASC device does not have the redox peaks at all scan rates. Figure 7b represents the specific capacity values of the Ni-foam@NiCu(CO3)(OH)2//graphene ASC and PAN@NiCu(CO3)(OH)2 electrode//graphene ASC from the discharge times, which are calculated to be 64 mAh g−1 and 126 mAh g−1 at 2 A g−1, respectively. This improvement in electrical performance is achieved by the electrical conductivity of the active materials and electrolyte penetration provided by the PAN nanofiber substrate to undergo a redox reaction. Therefore, high-speed performance is achieved of the development for high-performance faradaic capacitors. The retention values of the ASCs were measured with respect to the discharge time at a current density of 5 A g−1 in Fig. 7c. The assembled ASC of the Ni-foam@NiCu(CO3)(OH)2 and PAN@NiCu(CO3)(OH)2 electrodes retained 91.3 and 90.1% of their capacities, respectively, after 5000 cycles. The retention values of the ASC were similar after 5000 cycles, regardless of the substrate type. These properties indicate that the PAN@NiCu (CO3)(OH)2 electrode can facilitate a more stable cycle reaction when measuring the electrochemical value with the ASC than with the half-cell electrode. In addition, the energy density and power density were calculated using Eqs. (5) and (6) [64, 65]:
where E (Wh kg−1) is the energy density, \(I\)(A) is the applied current, \(\int V\mathrm{d}t\) is the galvanostatic discharge current area, \(M\) (g) is the active mass, P (W kg−1) is the power density, and \(\Delta t\) (s) is the discharge time. Figure 7d presents the ragone plot of the fabricated ASC devices. The PAN@NiCu(CO3)(OH)2 electrode ASC shows that the highest energy density with 90 W h kg−1 at a power density of 835 W kg−1 and current density of 2 A g−1. As the current density changes to 15 A g−1, the energy density decreases to 36 W h kg−1 at a power density of 9268 W kg−1. The PAN@NiCu(CO3)(OH)2 electrode achieved better energy and power densities than that of the Ni-foam@NiCu(CO3)(OH)2 electrode ACS, NiO//carbon [66], Ni(OH)2//carbon [67], and MnO2//carbon [68]. These results can aid the development of flexible and wearable faradaic capacitors with enhanced electrochemical performance by synthesizing PAN nanomeshes and modifying their surface through the growth of binary metal hydroxy-carbonates. Thus, PAN@NiCu(CO3)(OH)2 nanowires are promising materials of the positive electrode for high-performance supercapacitors.
Conclusions
Faradaic capacitors require a large specific surface area for redox reactions. To provide this large surface area, mesoporous NiCu(CO3)(OH)2 composites were grown on PAN nanofiber substrates using a hydrothermal method. PAN nanofibers were fabricated via the electrospinning method, giving a substrate with a nano-sized diameter and high specific surface area. Thus, the beneficial properties of high-performance SCs can arise from the structure as well as the material. The composite of NiCu(CO3)(OH)2 on PAN nanofibers provided a highly porous structure and a large surface area, which significantly improved the electrolyte penetration and electrical conductivity of the active materials, while providing a convenient electron diffusion path. The optimized PAN@NiCu(CO3)(OH)2 electrode showed an excellent maximum specific capacity of 870 mAh g−1 at 3 A g−1 and superior cycling stability with capacitance retention of 84.1% after 5000 cycles. An asymmetric supercapacitor was fabricated using the PAN@NiCu(CO3)(OH)2 composite as the cathode and commercial graphene as the anode. The ASC delivered a high energy density of 90 W h kg−1 at a power density of 835 W kg−1 at 2 A g−1 and maintained 36 W h kg−1 at a high power density of 9268 W kg−1 at 15 A g−1 with satisfactory cycling stability, retaining 90.1% of its capacitance after 5000 cycles. On the other hand, the previously studied NiCu(CO3)(OH)2//graphene ASC of Ni-foam substrate showed a relatively low energy density value with the energy density of 27 W h kg−1 at 2 A g−1. These results suggest that the optimized unique NiCu(CO3)(OH)2 composite on the PAN nanofiber substrate electrode is a potential material for use in wearable devices demanding flexibility. In conclusion, polymer and transition metal composite electrodes will potentially lead to further research and development of electrode industrialization and high-performance supercapacitors.
Experiment
Materials
The materials used in this study were Ni foam (110 PPI pore density, 320 g m−2 mass density), polyacrylonitrile (PAN), dimethylformamide (DMF), nickel nitrate hexahydrate (Ni(NO3)2·6H2O), copper nitrate trihydrate (Cu(NO3)2·3H2O), CO(NH2)2 (urea), and KOH (potassium hydroxide). All chemical reagents were used without further purification.
Electrode preparation
Ni foam as substrate: Two pieces of Ni foam (2 × 4 cm) were cleaned for 10 min by rinsing with 2.0 mol L−1 of HCl, washing with DI water and ethanol for 10 min each, and then drying at 50 °C in an oven for 12 h.
PAN nanofibers as substrates: PAN nanofibers were fabricated using the electrospinning method. The experimental conditions were set to PAN (0.72 g) and DMF (9 mL). As demonstrated in our previous experiments, this condition allows for the creation of an optimal nanofiber material. The applied voltage was approximately 15 kV, and the current was adjusted to a constant value. The PAN solution was poured into a syringe attached to a capillary tip with a 0.5 mm diameter, and the flow rate was uniform (20 mL h−1). The capillary tip and deposition position were kept constant at 15 cm. The substrate for PAN was not used separately, and the fabricated PAN nanofibers were directly used as the electrode substrate.
Fabrication of the Ni−Cu electrode: Optimized quantities of 6 mmol of Ni, 3 mmol of Cu, and 13 mmol of urea were placed in 50 mL of DI water and this solution was stirred for 10 min. This solution was moved to a 50 mL autoclave vessel. The Ni foam and PAN-nanofiber substrates were immersed in the aqueous solution separately and heated at 160 °C for 12 h via the hydrothermal method to synthesize NiCu(CO3)(OH)2. To remove surface impurities, the electrodes were washed several times with DI water and ethanol. And then, to remove adsorbed solvents, the samples were dried at 50 °C for 12 h. In the synthesis, urea is the source of both carbonate and hydroxyl anions, as indicated in Eqs. (7–9). The synthesis of NiCu(CO3)(OH)2 is as follows (7–9) [10, 27].
Material characterization
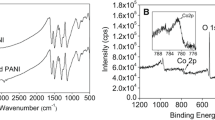
The phases of the samples were characterized using X-ray diffraction (XRD; STOE STADI MP), which were measured over a range of 5–60° (2θ). X-ray photoelectron spectroscopy (XPS; ESCALAB250, VG Scientifics) was examined to analyze the valence states of the NiCu(CO3)(OH)2 composite powder. The morphology analysis of the PAN@NiCu(CO3)(OH)2 nanowire electrodes was performed through scanning electron microscopy (SEM; FEI Strata Dual Beam 235). Transmission electron microscopy was measured using (TEM; JEOL JEM-2200 FS) with energy-dispersive X-ray spectroscopy.
Electrochemical measurements
All electrochemical tests were performed on an electrochemical workstation (VersaSTAT, Princeton Applied Research) using a three-electrode configuration using a 1 M KOH aqueous solution as the electrolyte at room temperature. NiCu(CO3)(OH)2 nanowire composites on PAN nanofibers were directly used as the cathode electrode, and Pt and Hg/HgO were used as the counter and reference electrodes, respectively. The cyclic voltammetry (CV) curves were plotted in a potential range between 0.15 and 0.6 V at different scan rates from 5 to 50 mV s−1. The galvanostatic charge–discharge (GCD) processes were evaluated by cycling the potential from 0 to 0.45 V at current densities of 3, 4, 5, 8, 10, and 15 A g−1.