Abstract
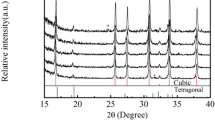
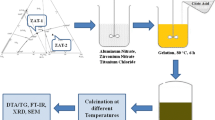
Glass-ceramic powders with a composition of Li2O · Al2O3 · 4SiO2 (LAS) have been synthesized by the sol-gel technique using LiOCH3, Al(OC2H5)3, Si(OC2H5)4, Ti(OC2H5)4, and Zr(OC2H5)4 as starting materials and the phase transformation behavior during calcination has been investigated. Differential thermal analysis (DTA), x-ray diffraction (XRD), and scanning electron microscopy (SEM) were utilized to determine the thermal behavior of the gels. Considering the LAS gels with 6.0 wt. % TiO2 and various wt. % ZrO2 content, and peak position of the β-spodumene phase formation in DTA curves was shifted to a higher temperature when the ZrO2 content was increased. The activation energy of β-spodumene crystallization was 283.8 kcal/mol for LAS gels with 6.0 wt. % TiO2 and 2.0 wt. % ZrO2. Unlike foregoing studies for LAS gels, during calcination of the dried LASTZ gels from 800 °C to 1200 °C neither β-eucryptite nor γ-spodumene was noted to be present. The crystallized phases comprised of β-spodumenes as the major phase and rutile (TiO2) together with zirconia (ZrO2) are precipitated as minor phases.
Similar content being viewed by others
References
P. A. Haas, Chem. Eng. Progress, Apr., 44 (1989).
D. W. Johnsor, Jr., Am. Ceram. Soc. Bull. 64, 1597 (1985).
H. Dislich, J. Non-Cryst. Solids 73, 599 (1985).
H. Schmidt, J. Non-Cryst. Solids 73, 681 (1985).
B. Samuneva, S. Jambazov, D. Lepkova, and Y. Dimitriev, Ceram. Int. 16, 355 (1990).
Ph. Colomban, Ceram. Int. 15, 23 (1989).
H. Murakami, S. Yaegashi, J. Nishino, Y. Shiohara, and S. Tanaka, Jpn. J. Appl. Phys. 29, 2715 (1990).
G. H. Beall and D. A. Duke, in Glass Science and Technology, edited by D.R. Uhlmann and N.J. Kreadl (Academic Press, New York, 1983), Vol. 1, pp. 403–445.
H. Suzuki, J. Takahashi, and H. Saito, J. Chem. Soc. Jpn. (10), 1312 (1991).
R. R. Tummala, J. Am. Ceram. Soc. 74, 895 (1991).
S. Knickerbocker, M. R. Tuzzolo, and S. Lawhorne, J. Am. Ceram. Soc. 72, 1873 (1989).
J. S. Yang, S. Sakka, T. Yoko, and H. Hozuka, J. Mater. Sci. 26, 1827 (1991).
H. Suzuki, J. Takahashi, and H. Saito, J. Chem. Soc. Jpn. (10), 1319 (1991).
G. Orcel and L.L. Hench, in Science of Ceramic Chemical Processing, edited by L. L. Hench and D.R. Ulrich (John Wiley and Sons, Inc., New York, 1986), pp. 224–230.
J. Phalippon, M. Prassas, and J. Zarzycki, J. Non-Cryst. Solids 48, 17 (1982).
R. Veltri and D. Scola, Powder Metall. Int. 21, 18 (1989).
H. Kobayashi, N. Ishibashi, T. Akiba, and T. Mitamura, Yogyo Kyokai Shi (J. Ceram. Soc. Jpn.) 98, 703 (1990).
C-Chen, F-S. Yen, and C-Y. Huang, Ceram. Int. 20, 379 (1994).
I. Artaki, M. Bradely, T. W. Zerda, J. Jonas, G. Orcel, and L. L. Hench, in Science of Ceramic Chemical Processing, edited by L. L. Hench and D.R. Ulrich (John Wiley and Sons, Inc., New York, 1983), pp. 73–80.
G. Orcel and L.L. Hench, J. Non-Cryst. Solids 79, 177 (1986).
J. Y. Hsu and R. F. Speyer, J. Am. Ceram. Soc. 73, 3585 (1990).
M.C. Wang, J. Ceram. Soc. Jpn. 102, 109 (1994).
V. W. Sack and H. Scheidler, Glastech. Ber. 39, 126 (1966).
A. Marotta, A. Buri, and G. L. Valenti, J. Mater. Sci. 13, 2483 (1978).
Author information
Authors and Affiliations
About this article
Cite this article
Lin, MH., Wang, MC. Phase transformation and characterization of TiO2 and ZrO2 addition in the Li2O-Al2O3-SiO2 gels. Journal of Materials Research 11, 2611–2615 (1996). https://doi.org/10.1557/JMR.1996.0328
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1996.0328