Abstract
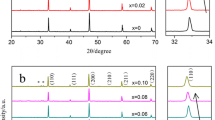
Al-Mo codoped Li7La3Zr2O12 ceramics with fine grain were prepared by sol-gel method. The influences of Al-Mo codo** on the structure, microstructure, and conductivity of Li7La3Zr2O12 were investigated by X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), and impedance spectroscopy. The cubic phase Li7La3Zr2O12 has been stabilized by partial substitution of Al for Li and Mo for Zr. Li6.6-3yAlyLa3Zr1.8Mo0.2O12 (0 ≤ y ≤ 0.1) has been sintered at 1040–1060 °C for 3 h. The liquid sintering facilitated its densification. The relative density of the composition with x = 0.075 was approximately 96.4%. Results indicated that the Al-Mo codoped LLZO synthesized by sol-gel method effectively lowered its sintering temperature, accelerated densification, and improved the ionic conductivity.









Similar content being viewed by others
References
Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R (2017) Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci 88:325–411
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed 34:437–440
Thangadurai V, Narayanan S, Pinzaru D (2014) Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem Soc Rev 43:4714–4727
Rao RP, Gu W, Sharma N, Peterson VK, Avdeev M, Adams S (2015) In situ neutron diffraction monitoring of Li7La3Zr2O12formation: toward a rational synthesis of garnet solid electrolytes. Chem Mater 27:2903–2910
Awaka J, Kijima N, Hayakawa H, Akimoto J (2009) Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J Solid State Chem 182:2046–2052
Geiger CA, Alekseev E, Lazic B, Fisch M, Armbruster T, Langner R, Fechtelkord M, Kim N, Pettke T, Weppner W (2010) Crystal chemistry and stability of “Li7La3Zr2O12” garnet: a fast lithium-ion conductor. Inorg Chem 50:1089–1097
Wagner R, Redhammer GJ, Rettenwander D, Tippelt G, Welzl A, Taibl S, Fleig J, Franz A, Lottermoser W, Amthauer G (2016) Fast Li-ion-conducting garnet-related Li7-3xFexLa3Zr2O12 with uncommon I4-3d structure. Chem Mater 28:5943–5941
Afyon S, Krumeich F, Rupp JLM (2015) A shortcut to garnet-type fast Li-ion conductors for all solid state batteries. J Mater Chem A 3:18636–18648
Robben L, Merzlyakova E, Heitjans P, Gesing TM (2016) Symmetry reduction due to Gallium substitution in the garnet Li6.43(2)Ga0.52(3)La2.67(4)Zr2O12. Acta Cryst E72:287–289
Wu JF, Chen EY, Yu Y, Liu L, Wu Y, Pang WK, Peterson VK, Guo X (2017) Gallium-doped Li7La3Zr2O12 garnet-type electrolytes with high lithium-ion conductivity. ACS Appl Mater Interfaces 9:1542–1552
Deviannapoorani C, Shankar LS, Ramakumar S, Murugan R (2016) Investigation on lithium ion conductivity and structural stability of yttrium-substituted Li7La3Zr2O12. Ionics 22:1281–1289
Yan B, Kotobuki M, Liu (2016) Ruthenium doped cubic-garnet structured solid electrolyte Li7La3Zr2−xRuxO12. Mater Technol 31:1–5
Trofimov AA, Li C, Brinkman KS, Jacobsohn LG (2017) Luminescence investigation of Ce incorporation in garnet-type Li7La3Zr2O12. Opt Mater 68:7–10
Song S, Sheptyakov D, Korsunsky AM, Duong HM, Lu L (2016) High Li ion conductivity in a garnet-type solid electrolyte via unusual site occupation of the do** Ca ions. Mater Des 93:232–237
Shao C, Yu Z, Liu H, Zheng Z, Sun N, Diao C (2017) Enhanced ionic conductivity of titanium doped Li7La3Zr2O12 solid electrolyte. Electrochim Acta 225:345–349
Ohta S, Kobayashi T, Asaoka T (2011) High lithium ionic conductivity in the garnet-type oxideLi7−xLa3(Zr2−x,Nbx)O12 (x=0 - 2). J Power Sources 196:3342–3345
Janani N, Ramakumar S, Kannan S, Murugan R (2015) Optimization of Lithium content and sintering aid for maximized Li+ conductivity and density in Ta-doped Li7La3Zr2O12. J Am Ceram Soc 98:2039–2046
Ramakumar S, Satyanarayana L, Manorama SV, Murugan R (2013) Structure and Li+ dynamics of Sb-doped Li7La3Zr2O12 fast lithium ion conductors. Phys Chem Chem Phys 15:11327–11338
**a W, Xu B, Duan H, Guo Y, Kang H, Li H, Liu H (2016) Ionic conductivity and air stability of Al-doped Li7La3Zr2O12 sintered in Alumina and Pt crucibles. Appl Mater Interfaces 8:5335–5342
Rettenwander D, Welzl A, Cheng L, Fleig J, Musso M, Suard E, Doeff MM, Redhammer GJ, Amthauer G (2015) Synthesis, crystal chemistry, and electrochemical properties of Li7-2xLa3Zr2-xMoxO12 (x=0.1-0.4): Stabilization of the cubic garnet polymorph via substitution of Zr4+ by Mo6+. Inorg Chem 54:10440–10449
Deviannapoorani C, Dhivya L, Ramakumar, Murugan R (2013) Lithium ion transport properties of high conductive tellurium substituted Li7La3Zr2O12 cubic lithium garnets. J Power Sources 240:18–25
** Y, McGinn PJ (2011) Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J Power Sources 196:8683–8687
Rangasamy E, Wolfenstine J, Sakamoto J (2012) The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 206:28–32
Düvel A, Kuhn A, Robben L, Wilkening M, Heitjans P (2012) Mechanosynthesis of solid electrolytes: preparation, characterization, and Li ion transport properties of garnet-type Al-doped Li7La3Zr2O12 crystallizing with cubic symmetry. J Phys Chem C 116:15192–15202
El-Shinawi H, Paterson GW, MacLaren DA, Cussen EJ, Corr SA (2017) Low-temperature densification of Al-doped Li7La3Zr2O12: a reliable and controllable synthesis of fast-ion conducting garnets. J Mater Chem A 5:319–329
Kumazaki S, Iriyama Y, Kim KH, Murugan R, Tanabe K, Yamamoto K, Hirayama T, Ogumi Z (2011) High lithium ion conductive Li7La3Zr2O12 by inclusion of both Al and Si. J Electrochem Commun 13:509–512
Rettenwander D, Redhammer G, Preishuber-Pflugl F, Cheng L, Miara L, Wagner R, Welzl A, Suard E, Doeff MM, Wilkening M, Fleig J, Amthauer G (2016) Structural and electrochemical consequences of Al and Ga cosubstitution in Li7La3Zr2O12solid electrolytes. Chem Mater 28:2384–2392
Wang D, Zhong G, Dolotko O, Li Y, Mcdonald M, Mi J, Fu R, Yang Y (2014) The synergistic effects of Al and Te on the structure and Li+-mobility of garnet-type solid electrolytes. J Mater Chem A 2:20271–20279
Mukhopadhyay S, Thompson T, Sakamoto J, Huq A, Wolfenstine J, Allen JL, Bernstein N, Stewart DA, Johannes MD (2015) Structure and stoichiometry in supervalent doped Li7La3Zr2O12. Chem Mater 27:3658–3665
Allen JL, Wolfenstine J, Rangasamy E, Sakamoto J (2012) Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J Power Sources 206:315–319
Liu K, Ma JT, Wang CA (2014) Excess lithium salt functions more than compensating for lithium loss when synthesizing Li6.5La3Ta0.5Zr1.5O12 in alumina crucible. J Power Sources 260:109–114
Ren Y, Deng H, Chen R, Shen Y, Lin Y, Nan CW (2015) Effects of Li source on microstructure and ionic conductivity of Al-contained Li6.75La3Zr1.75Ta0.25O12ceramics. J Eur Ceram Soc 35:561–572
Kokal I, Somer M, Notten PHL, Hintzen HT (2011) Sol-gel synthesis and lithium ion conductivity of Li7La3Zr2O12 with garnet-related type structure. Solid State Ionics 185:42–46
Shimonishi Y, Toda A, Zhang T, Hirano A, Imanishi N, Yamamoto O, Takeda Y (2011) Synthesis of garnet-type Li7-xLa3Zr2O12-1/2x and its stability in aqueous solutions. Solid State Ionics 183:48–53
Janani N, Deviannapoorani C, Dhivya L, Murugan R (2014) Influence of sintering additives on densification and Li+ conductivity of Al doped Li7La3Zr2O12 lithium garnet. RSC Adv 4:51228–51238
Takano R, Tadanaga K, Hayashi A, Tatsumisago M (2014) Low temperature synthesis of Al-doped Li7La3Zr2O12 solid electrolyte by a sol-gel process. Solid State Ionics 255:104–107
Tadanaga K, Takano R, Ichinose T, Mori S, Hayashi A, Tatsumisago M (2013) Low temperature synthesis of highly ion conductive Li7La3Zr2O12-Li3BO3 composites. J Electrochem Commun 33:51–54
Li Y, Wang Z, Li C, Cao Y, Guo X (2014) Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J Power Sources 248:642–646
Wolfenstine J, Sakamoto J, Allen JL (2012) Electron microscopy characterization of hot-pressed Al substituted Li7La3Zr2O12. J Mater Sci 47:4428–4431
Suzuki Y, Kami K, Watanabe K, Watanabe A, Saito N, Ohnishi T, Takada K, Sudo R, Imanishi N (2015) Transparent cubic garnet-type solid electrolyte of Al2O3-doped Li7La3Zr2O12. Solid State Ionics 278:172–176
Amores M, Ashton TE, Baker PJ, Cussen EJ, Corr SA (2016) Fast microwave-assisted synthesis of Li-stuffed garnets and insights into Li diffusion from muon spin spectroscopy. J Mater Chem A 4:1729–1736
Omori M (2000) Sintering, consolidation, reaction and crystal growth by the spark plasma system (SPS). Mater Sci Eng A 287:183–188
Groza JR, Zavaliangos A (2000) Sintering activation by external electrical field. Mater Sci Eng A 287:171–177
Nygren M, Shen Z (2003) On the preparation of bio-, nano- and structural ceramics and composites by spark plasma sintering. Solid State Sci 5:125–131
Orru R, Licheri R, Locci AM, Cincotti A, Cao G (2009) Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater Sci Eng R 63:127–287
Räthel J, Herrmann M, Beckert W (2009) Temperature distribution for electrically conductive and non-conductive materials during field assisted sintering (FAST). J Eur Ceram Soc 29:1419–1425
Baek SW, Lee JM, Kim TY, Song MS, Park Y (2014) Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J Power Sources 249:197–206
Ni JE, Case ED, Sakamoto JS, Rangasamy E, Wolfenstine JB (2012) Room temperature elastic moduli and Vickers hardness of hot-pressed LLZO cubic garnet. J Mater Sci 47:7978–7985
Oudenhoven JFM, Baggetto L, Notten PHL (2011) Allsolidstate Lithiumion microbatteries: A review of various three-dimensional concepts. Adv Energy Mater 1:10–33
Mccloskey BD (2015) Attainable gravimetric and volumetric energy density of Li-S and Li ion battery cells with solid separator-protected Li metal anodes. J Phys Chem Lett 6:4581–4588
Cheng L, Hou H, Lux S, Kostecki R, Davis R, Zorba V, Mehta A, Doeff M (2017) Enhanced lithium ion transport in garnet-type solid state electrolytes. J Electroceram 38:168–175
Kim Y, Jo H, Allen JL, Choe H, Wolfenstine J, Sakamoto J, Pharr G (2016) The effect of relative density on the mechanical properties of Hot-pressed cubic Li7La3Zr2O12. J Am Ceram Soc 99:1367–1374
Il’ina EA, Andreev OL, Antonov BD, Batalov NN (2012) Morphology and transport properties of the solid electrolyte Li7La3Zr2O12 prepared by the solid-state and citrate-nitrate methods. J Power Sources 201:169–173
Liu X, Li Y, Yang T, Cao Z, He W, Gao Y, Liu J, Li G, Li Z (2017) High lithium ionic conductivity in the garnet-type oxide Li7−2xLa3Zr2−xMoxO12 (x=0-0.3) ceramics by sol-gel method. J Am Ceram Soc 100:1527–1533
Bottke P, Rettenwander D, Schmidt W, Amthauer G, Wilkening M (2015) Ion dynamics in solid electrolytes: NMR reveals the elementary steps of Li+ hop** in the garnet Li6.5La3Zr1.75Mo0.25O12. Chem Mater 27:6571–6582
Gao YX, Wang XP, Lu H, Zhang LC, Ma L, Fang QF (2016) Mechanism of lithium ion diffusion in the hexad substitutedLi7La3Zr2O12 solid electrolytes. Solid State Ionics 291:1–7
Chen F, Li J, Zhang Y, Yang D, Shen Q, Zhang L (2017) Effect of Mo6+ substitution on microstructure and lithium ionic conductivity of garnet-type Li7La3Zr2O12 solid electrolytes by field assisted sintering technology. In: Meyers M. et al. (eds) Proceedings of the 3rd pan american materials congress. The minerals, metals & materials series. Springer, Cham, pp. 115–122
Larson AC, Von Dreele RB (1994) General structure analysis system (GSAS), report LAUR. Los Alamos National Laboratory, Los Alamos, pp. 86–748
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34:210–213
Larraz G, Orera A, Sanjuán ML (2013) Cubic phases of garnet-type Li7La3Zr2O12: the role of hydration. J Mater Chem A 1:11419–11428
Julien C (2000) 4-Volt cathode materials for rechargeable lithium batteries wet-chemistry synthesis, structure and electrochemistry. Ionics 6:30–46
Julien C, Massot M (2003) Lattice vibrations of materials for lithium rechargeable batteries I. Lithium manganese oxide spinel. Mater Sci Eng B 97:217–230
Dhivya L, Murugan R (2014) Effect of simultaneous substitution of Y and Ta on the stabilization of cubic phase, microstructure, and Li+ conductivity of Li7La3Zr2O12 lithium garnet. ACS Appl Mater Interfaces 6:17606–17615
Tietz F, Wegener T, Gerhards MT, Giarola M, Mariotto G (2013) Synthesis and Raman micro-spectroscopy investigation of Li7La3Zr2O12. Solid State Ionics 230:77–82
Yang T, Li Y, Wu W, Cao Z, He W, Gao Y, Liu J, Li G (2018) The synergistic effect of dual substitution of Al and Sb on structure andionic conductivity of Li7La3Zr2O12 ceramic. Ceram Int 44:1538–1544
Moser M, Klimm D, Ganschow S, Kwasniewski A, Jacobs K (2008) Re-determination of the pseudobinary system Li2O-MoO3. Cryst Res Technol 43:350–354
Byker HJ, Eliezer I, Eliezer N, Howald RA (1979) Calculation of a Phase Diagram for the LiO0.5-A10.5 System. J Phys Chem C 83:2349–2355
Rosero-Navarro NC, Yamashita T, Miura A, Higuchi M, Tadanaga K (2017) Effect of sintering additives on relative density and Li-ion conductivity of Nb-doped Li7La3ZrO12 solid electrolyte. J Am Ceram Soc 100:276–285
Zhang LC, Yang JF, Gao YX, Wang XP, Fang QF, Chen CH (2017) Influence of Li3BO3 additives on the Li+ conductivity and stability of Ca/Ta-substituted Li6.55(La2.95Ca0.05)(Zr1.5Ta0.5)O12 electrolytes. J Power Sources 355:69–73
Li Y, Han JT, Wang CA, **e H, Goodenough JB (2012) Optimizing Li+ conductivity in a garnet framework. J Mater Chem 22:15357
Li C, Liu Y, He J, Brinkman KS (2017) Ga-substituted Li7La3Zr2O12: An investigation based on grain coarsening in garnet-type lithium ion conductors. J Alloys Compd 695:3744–3752
Funding
This study was supported by the Program for National Natural Science Foundation of China (No. 51562029), Program for Key Laboratory of Inorganic Function Material and Device, Chinese Academy of Sciences (KLIFMD-2011-01), and Program for Young Talents of Science and Technology in University of Inner Mongolia Autonomous Region (No. NJYT-17-A08).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Y., Yang, T., Wu, W. et al. Effect of Al-Mo codo** on the structure and ionic conductivity of sol-gel derived Li7La3Zr2O12 ceramics. Ionics 24, 3305–3315 (2018). https://doi.org/10.1007/s11581-018-2497-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2497-3