Abstract
Background
We sought to determine the significance of Ki-67, one of the tumor cell proliferation markers, as a useful prognostic factor in early breast cancer.
Methods
A total of 1080 consecutive patients with stage I or II breast cancer that underwent surgery between 1998 and 2003 were enrolled. Patients were categorized on the basis of the 2007 St. Gallen consensus and Adjuvant! Online. The expression of Ki-67 in the tumor was assayed by immunohistochemistry (cutoff value, 10%).
Results
Univariate analysis determined that tumor size, lymph node involvement, histologic grade, estrogen receptor, progesterone receptor, bcl-2, and Ki-67 (≥10%) were statistically significant for both overall survival (OS) and distant metastasis-free survival (DFS). Of these factors, lymph node involvement and high Ki-67 expression were identified as independent prognostic factors for OS and DFS on the basis of multivariate analysis. The survivals of intermediate- and high-risk groups according to 2007 St. Gallen consensus were further separated by Ki-67 expression level (5-year DFS rate = 91.9% vs. 86.3% for Ki-67 < 10% and ≥10%, respectively in intermediate-risk group (P = .01); 5-year DFS rate = 82.5% vs. 61.4% for Ki-67 < 10% and ≥10%, respectively in high-risk group (P = .01)). The survivals of low- and high-risk groups according to Adjuvant! Online were further separated by Ki-67 expression level (5-year DFS rate = 97.8% vs. 89.5% for Ki-67 < 10% and ≥10%, respectively in low-risk group (P = .02); 5-year DFS rate = 9.4% vs. 82.6% for Ki-67 < 10% and ≥10% in high-risk group (P = .005)).
Conclusions
Ki-67 is an independent prognostic factor for DFS and OS in early breast cancer and can provide additional prognostic information on the risk stratification with the use of the 2007 St. Gallen consensus and Adjuvant! Online.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Among patients with early breast cancer, approximately 20% to 50% of patients who received only curative surgery will experience disease recurrence and die of systemic disease. These patients have obtained statistically significant improvements of disease-free survival and overall survival with the extensive use of adjuvant systemic therapies.1 For this reason, many patients with early breast cancer have received systemic treatment.
The St. Gallen consensus is a currently available guideline for adjuvant treatment of breast cancer patients that divides breast cancer patients with early breast cancer into three risk groups (low-risk group, intermediate-risk group, and high-risk group) on the basis of clinicopathologic factors such as tumor size, nodal status, tumor grade, hormone receptor, human epidermal growth factor receptor-2 (HER-2) expression, and age at diagnosis.2 However, this consensus is not satisfactory for selecting subsets of patients who are more likely to benefit from adjuvant chemotherapy and predicting prognosis of patients with early breast cancer.3,4
Adjuvant! Online (http://www.adjuvantonline.com/) is one of the tools to quantitatively estimate the prognosis of patients with breast cancer and the benefit of adjuvant systemic therapy. It is based on a 10-year observed survival for women age 36 to 69 years with breast cancer between 1998 and 1992 recorded in the Surveillance, Epidemiology, and End Results Program registry in the United States.5 Estimates of the efficacy of the use of adjuvant tamoxifen and chemotherapy were derived from the Early Breast Cancer Trialists’ Collaborative Group. Although Adjuvant! Online is a useful method to predict the prognosis of disease and benefit of treatment, it needs more validation and modification because it is too optimistic for subgroups enriched with adverse prognostic factors, such as progesterone receptor (PgR) negativity and HER-2 overexpression.6
To identify high-risk patients for disease recurrence, many prognostic factors have been described over the last few decades. Tumor size, nodal status, and histological grade are known as prognostic factors of breast cancer and are used for identifying patients who might benefit from adjuvant chemotherapy.7 Among clinical variables, age at diagnosis is a validated prognostic marker. Especially patients younger than 35 years of age had shown unfavorable prognosis.8 For the last few years, as attention on the molecular features of tumors has been increased, several biomarkers have been demonstrated as prognostic factors. For example, hormone receptors have been used for predicting prognosis and selecting therapeutic modalities such as the administration of tamoxifen. Recently, expression of HER-2, an oncogenetic transmembrane growth factor receptor, has been demonstrated as a prognostic surrogate and might be used to modify the previous St. Gallen consensus and help select therapy such as trastuzumab.9 Although many prognosticators have been reported, the determination of more accurate and clinically available markers remain a major challenge.10
Because rapid tumor proliferation is a critical feature for tumor aggressiveness, proliferation markers have been extensively evaluated as prognostic tools in breast cancer.11 Ki-67 is a cell proliferation-associated antigen that is expressed in all stages of the cell cycle except G.0 12 Determination of the percentage of Ki-67 expression has become a standard method to assess the proliferative activity of tumor cells.13 Several investigations have reported that Ki-67 overexpression is related to an unfavorable outcome for breast cancer.14,15 In recent studies of gene-expression profiling for breast cancer, Ki-67 has been identified as one of the selected genes used to predict disease recurrence.16 Despite the many articles published that analyze the prognostic role of Ki-67 in early breast cancer, it is still not considered to be an available prognosticator in clinical practice.10,17–19
The purpose of the current study was to identify prognostic factors in early breast cancer and to determine the importance of Ki-67 as a prognostic factor in risk groups that are based on the St. Gallen consensus and Adjuvant! Online.
Materials and Methods
Patients
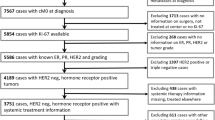
The study population consisted of 1080 consecutive patients with a pathologic diagnosis of stage I or stage II breast cancer based on the American Joint Committee on Cancer cancer staging manual. Patients were enrolled between January 1998 and December 2003 at the Department of Surgery, Seoul National University College of Medicine. Local therapies consisted of mastectomy or breast-conserving surgery. Six hundred seventy patients (62.0%) underwent mastectomy, and 410 patients (38.0%) underwent breast-conserving surgery. Adjuvant radiotherapy followed after breast-conserving surgery, and 799 patients (74.0%) received adjuvant chemotherapy. The regimens were a combination of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) or an anthracycline-containing regimen. Adjuvant hormone therapy was administered when estrogen receptor (ER) or PgR was positive by immunohistochemistry (IHC). None of patients was treated with trastuzumab as adjuvant therapy. Patients diagnosed with pure in-situ carcinoma or who showed distant metastasis at the time of diagnosis were excluded from the analysis. Patients who underwent neoadjuvant chemotherapy also were excluded.
IHC
The routinely formalin-fixed, paraffin-embedded tissue blocks were sectioned at a 4-μm thickness and processed for IHC. Paraffin was removed from tissue sections with xylene. The sections were rehydrated with graded ethanol and immersed in Tris-buffered saline. Representative sections were immunostained, and >10 randomly chosen high-power fields were examined under an optical microscope.
The companies that supplied the primary antibodies and the dilution factors used were as follows; ER (1DO5; Dako; 1:50), PgR (PgR636; Dako; 1:50), p53 (DO-7A; Dako; 1:1200), HER-2 (CB11; Novocastra Laboratories, Newcastle-Upon-Tyne, UK; 1:200), bcl-2 (124; Dako; 1:50), and Ki-67 (MIB-1; Dako; 1:800). Biotinylated anti-mouse antibody was used as a secondary antibody, and streptavidin horseradish peroxidase (Zymed Laboratories, San Francisco, CA) methods were used following the instructions provided by the manufacturer. Finally, the sections were counterstained in Mayer’s hematoxylin, dehydrated, and cleared, and the sections were mounted for examination.
The slides were examined and scored by one pathologist. A cutoff value of ≥10% of the positively stained nuclei was used to define ER and PgR positivity. Only cytoplasmic staining was scored as positive for bcl-2, regardless of the intensity of the stained cells. Membranous staining for HER-2 was scored as the following: 0, faint incomplete staining in ≤10% of the cells; 1+ , faint incomplete staining in >10% of the cells; 2+, weak to moderate complete staining in >10% of the cells; 3+, strong complete staining in >10% of the cells. Cells stained for Ki-67 and p53 were counted and expressed as a percentage. The percentage was determined by the number of Ki-67-positive cells among the total number of total counted tumor cells.
St. Gallen Consensus
Patients were divided into three groups in accordance with the St. Gallen consensus published in 2007.20 The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis.21 ER, PgR, and HER-2 expression was defined by IHC. The low-risk group consisted of patients with node-negative disease who had all of the following features: tumor size ≤2 cm, tumor grade 1 or 2, expression of ER and/or PgR, HER-2 neither overexpressed nor amplified, and age ≥35 years. Patients with node-positive and ER and PgR absent, or HER-2 gene overexpressed or amplified were classified into the high-risk group. In this study, patients with tumor grade 2 without another risk factor were included in the low-risk group. Tumors with 3+ in IHC were defined as positive for HER-2 overexpression.
Adjuvant! Online
We calculated the 10-year survival probability of patients by Adjuvant! software version 8.0 (http://www.adjuvantonline.com/), which is based on the patient’s age, tumor size and grade, tumor ER status, and nodal status. Patients were divided into two subgroups (low and high) by using two cutoffs that were decided by consensus of the translational research established by the Breast International Group (TRANSBIG) consortium members (http://www.breastinternationalgroup.org/). The low-risk group was defined as patients with a 10-year survival probability of at least 88% with positive expression of ER and of at least 92% with negative expression of ER.
Statistical Methods
The study end points were distant metastasis-free survival (DFS) and overall survival (OS). The DFS period was defined as the interval from the date of operation to the date of the first observation of a distant metastasis or the last follow-up date without evidence of distant metastasis. The OS period was calculated from the date of operation to the date of death or the last follow-up date when the patients was alive. Survival rates were calculated by the Kaplan-Meier method, and comparisons between groups were made by the log rank test. For multivariate analysis, Cox regression analysis was used. Statistical analysis was performed by SPSS 13.0 for Windows (SPSS, Chicago, IL).
Ethics
This study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital; it complied with the recommendations of the Declaration of Helsinki for biomedical research involving human subjects.
Results
Clinicopathological Characteristics of the Patients
One thousand eighty patients with stage I and II breast cancer were enrolled onto the study. The median age at diagnosis was 47 years (range, 21–83 years). The median follow-up duration was 43.9 months (range, 13–117 months). One hundred ten patients (10.2%) experienced disease recurrence, and 37 patients (3.4%) died. The clinicopathological features of the enrolled patients are listed in Table 1.
Univariate and Multivariate Analysis
Table 1 shows the result of univariate analysis for DFS. Statistically significant prognostic factors for DFS were age at diagnosis, tumor size, lymph node involvement, histologic grade, adjuvant chemotherapy, ER, PgR, HER-2, bcl-2, and Ki-67. However, menopausal status, nuclear grade, p53, and adjuvant hormone therapy were not statistically significant. OS was related to tumor size, lymph node involvement, histologic grade, ER, PgR, p53, bcl-2, and Ki-67. However, age at diagnosis, menopausal status, nuclear grade, and HER-2 were not statistically significant factors.
Multivariate analysis was performed for the DFS and OS by using statistically significant variables as determined by univariate analysis. Age at diagnosis (hazard ratio [HR] 1.81; 95% confidence interval [95% CI], 1.02–3.19), lymph node involvement (HR 2.60; 95% CI, 1.71–3.92), and Ki-67 overexpression (HR 1.84; 95% CI, 1.17–2.90) were identified as independent factors significantly associated with recurrence (Table 2). Lymph node involvement (HR 3.35; 95% CI, 1.70–6.60) and Ki-67 overexpression (HR 2.49; 95% CI, 1.19-5.20) correlated with unfavorable OS (Table 3).
Association Between Ki-67 Expression and Other Prognostic Markers
The association between Ki-67 expression and other prognostic markers were assessed for the patients with early breast cancer (Table 4). Ki-67 expression was found to be correlated with tumor size, tumor grade, p53 expression, and HER-2 expression (P < .001). Expression of ER, PgR, and bcl-2 expression were inversely correlated with Ki-67 expression (P < .001). However, there was no relation between Ki-67 and nodal status. The patients with Ki-67 overexpression were treated with chemotherapy much more than the patients without Ki-67 overexpression (P < .001).
DFS According to Ki-67 Expression and Hormone Receptor Status
Considering the inverse correlation of Ki-67 and hormone receptor, hormone receptor-positive and -negative subgroups were analyzed separately for DFS rate according to Ki-67 expression. In tumors with hormone receptor, the 5-year DFS rate was 94.8% with Ki-67 < 10% tumor and 86.0% with Ki-67 ≥ 10% tumor (P = .004, Fig. 1a). Patients without hormone receptor expression showed a statistically significant difference of DFS with Ki-67 expression (5-year DFS rate, 89.4% for Ki-67 < 10% tumor vs. 84.7% for Ki-67 ≥ 10% tumor, P = .03, Fig. 1b).
DFS According to Ki-67 Expression and Chemotherapy Regimens
Considering the variability of chemotherapy regimens, survivals by Ki-67 expression were analyzed separately according to chemotherapy regimens. Two hundred eighty-one patients (26.0%) did not receive chemotherapy, 548 patients (50.7%) were treated with CMF, and 251 patients (23.3%) were treated with anthracycline-containing chemotherapy. In patients who did not receive chemotherapy, the survival was worse when Ki-67 expression was high in the tumor, although it is nonsignificant (5-year DFS rate, 98.7% for Ki-67 < 10% tumor vs. 95.7% for Ki-67 ≥ 10% tumor, P = .21, Fig. 2a). Patients treated with CMF showed a significant difference of DFS according to Ki-67 expression (5-year DFS rate, 90.4% for Ki-67 < 10% tumor vs. 83.2% for Ki-67 ≥ 10% tumor, P = .01, Fig. 2b). In subgroup treated with anthracycline- containing chemotherapy, there was a significant difference of DFS according to Ki-67 expression (5-year DFS rate, 90.2% for Ki-67 < 10% tumor vs. 78.8% for Ki-67 ≥ 10% tumor, P = .003, Fig. 2c).
DFS of Risk Groups Based on the St. Gallen Classification and Adjuvant! Online
Patients were divided into three risk groups (low, intermediate, and high) according to the 2007 St. Gallen consensus. We were able to classify 1066 patients (98.7%). A total of 196 patients (18.4%) were classified as low risk, 786 patients (73.7%) as intermediate risk, and 84 patients (7.9%) as high risk. When the DFS rates of subgroups were compared, high-risk patients showed unfavorable prognosis compared with intermediate- and low-risk patients (5-year DFS, 71.4% for high-risk group vs. 90.1% for intermediate-risk group vs. 97.5% for low-risk group, P < .001).
On the basis of Adjuvant! Online, we found the median 10-year survival probability of enrolled patients was 80% (range, 35%-99%). Two hundred forty-eight patients (23.0%) were included in the low-risk group and 832 patients (77.0%) in the high-risk group. There was a statistically significant difference in DFS between the two risk groups (5-year DFS, 87.6% for high-risk group vs. 97.2% for low-risk group, P = .001).
Comparison of DFS According to Ki-67 Expression in the St. Gallen Classification and Adjuvant! Online
We reclassified three risk groups of the St. Gallen consensus according to Ki-67 expression and analyzed the DFS rate of each subgroup. In the low-risk group, there was no difference of DFS rate with or without Ki-67 overexpression (Fig. 3a). The 5-year DFS rate was 91.9% with Ki-67 < 10% tumor and 86.3% with Ki-67 ≥ 10% tumor among intermediate-risk patients (P = .01, Fig. 3b). In high-risk patients, there was significant difference of DFS by Ki-67 expression (5-year DFS rate, 82.5% for Ki-67 < 10% tumor vs. 61.4% for Ki-67 ≥ 10% tumor, P = .01, Fig. 3c).
For two subgroups of Adjuvant! Online, we compared the DFS rate according to Ki-67 expression. In both subgroups, there were statistically significant differences of DFS rates with or without Ki-67 overexpression. The 5-year DFS rate was 97.8% with Ki-67 < 10% tumor and 89.5% with Ki-67 ≥ 10% tumor in low-risk patients (P = .02, Fig. 4a). In the high-risk subgroup, DFS rates by Ki-67 expression were significantly different (5-year DFS rate, 90.4% for Ki-67 < 10% tumor vs. 82.6% for Ki-67 ≥ 10% tumor, P = .005, Fig. 4b).
Discussion
The current study was designed to evaluate the importance of Ki-67 as a prognostic factor in early breast cancer. We have demonstrated that Ki-67 is an independent prognostic marker for DFS and OS of early breast cancer. The adverse effect of Ki-67 overexpression for both DFS and OS was observed in the subgroups lymph node-negative and lymph node-positive patients as well as in the overall population. In addition, analysis that used a combination of Ki-67 status and St. Gallen consensus data confirmed that Ki-67 expression had prognostic power in the intermediate-risk and high-risk groups.
Several studies have evaluated the correlation between Ki-67 expression and clinical outcome (DFS and/or OS).11,18,22–26 Although the findings are controversial, many studies have reported the independence of Ki-67 expression as a prognostic marker. In a meta-analysis of Ki-67 in early breast cancer, Ki-67 overexpression resulted in unfavorable survival in lymph node-negative and lymph node-positive patients.17 This recent study showed the same result in a subgroup analysis for nodal status. In a study of protein expression profiling that used IHC with a tissue microarray, Jacquemier et al. delineated protein clusters associated with ER and with tumor proliferation, and the investigators identified a set of 21 proteins whose combined expression was statistically significantly correlated with DFS.27 In multivariate analysis, the 21-protein signature, tumor size, nodal status, and Ki-67 expression have been determined to be independent prognosticators.27
Genomic studies have also demonstrated that Ki-67 expression affects the prognosis of breast cancer. Paik et al. quantified the likelihood of distant metastases in patients with lymph node-negative breast cancer by using a gene-expression assay and an algorithm for calculation recurrence scores.16 A panel of 21 genes included the estrogen group, the proliferation group, and the HER-2 group. Ki-67 was a representative gene of the proliferation group. In a 76-gene signature of the study by Wang et al. and 70-gene profile by van de Vijver et al., genes that were involved in cell proliferation influenced distant metastasis of breast cancer.28,29 However, these gene assays are difficult to apply in the clinical setting because of its high cost and its requirement for fresh tissue. Therefore, Ki-67 expression determination by immunoassay could be considered a convenient method to evaluate tumor proliferation in clinical practice, and subsequently for treatment decisions and prognosis prediction.
Because tumor proliferation is a central cause of disease recurrence and distant metastases, many researchers have sought a way to determine the proliferative rate in neoplasms. Previously established methods include the use of the thymidine-labeling index, bromodeoxyuridine incorporation, and S-phase fraction measurement.30–32 However, the use of antibodies to Ki-67 is known to be the most reliable and simplest method to assess tumor proliferation. In particular, the monoclonal antibody MIB-1, which has been widely used in clinical practice and in this study, is superior to other antibodies because it is readily detectable in a paraffin-embedded section and provides good correlation of the Ki-67 expression level in frozen sections.33
The recommendation for the cutoff of the level of Ki-67 expression affecting prognosis is controversial. Previous investigations about Ki-67 expression and survival have used 5%, 10%, 20%, 25%, or a median value as a cutoff value.22–26,34,35 In this study, we calculated survival for different cutoff values of Ki-67 expression (5%, 10%, 20%, 25%, and 50%) and concluded that 10% as cutoff provided the best prognosis-prediction results. In a study about the clinical implication of the Ki-67 cutoff value, the choice of Ki-67 cutoff may depend on the clinical objective.36 If Ki-67 expression is used to exclude patients with slowly proliferating tumors from chemotherapy protocols, a cutoff of 10% will help to avoid overtreatment. If Ki-67 expression is used to identify tumors that are sensitive to chemotherapy, it seems preferable to set the cutoff at 25%. For this reason, when we selected high-risk subsets of patients for adjuvant chemotherapy, it was reasonable to select 10% as a cutoff value in this study.
There were some explanations for the prognostic role of Ki-67 expression in breast cancer. Some investigators have reported an association between Ki-67 expression and other prognostic variables, e.g., tumor grade, nodal status, tumor size, age at diagnosis, hormone receptor status, and epidermal growth factor receptor expression.19,37,38 This study demonstrated that Ki-67 expression was related with tumor size, tumor grade, and p53 expression, and had an inverse association with favorable prognostic markers such as hormone receptor and bcl-2 expression. Therefore, it was necessary to adjust the other prognostic variables to evaluate the independent effect of Ki-67 expression. This current study confirmed that Ki-67 expression was an independent prognostic marker by multivariate analysis when other associated factors were used as covariates.
The St. Gallen consensus, one of several currently available criteria for adjuvant chemotherapy in breast cancer, could reflect the prognosis of breast cancer in this study. The survival comparison of the three risk groups showed a statistically significant difference. However, the St. Gallen consensus has several key problems. These problems could lead to overtreatment or undertreatment in clinical practice.3,39,40 One problem is that the number of patients in the low-risk group is too small. In this study, only 196 patients (18.4%) were classified in the low-risk group. In studies of the application of the St. Gallen consensus for lymph node-negative patients, only 10% of patients were classified as low risk.3,9 Because grade 2 tumors were included in the low-risk group in this study, the percentage of the low-risk group was larger than when only grade 1 tumors were included in low-risk group. If the St. Gallen consensus is applied more strictly, only a few patients could avoid adjuvant chemotherapy. The other defect of the St. Gallen consensus is that the spectrum of the intermediate-risk group was too broad. Seven hundred eighty-six patients (73.7%) were classified as intermediate risk. However, the prognoses of patients in this group were heterogeneous. For example, the prognosis of patients with Ki-67 < 10% in the intermediate-risk group was not different with that of patients with Ki-67 ≥ 10% in the low-risk group (5-year DFS rate 91.9% vs. 93.3%).
Adjuvant! Online could provide estimates of survival probability with and without adjuvant systemic therapy. Several studies have validated the use of this software and have used it for validation of other prediction models.6,41 However, Adjuvant! Online has a limitation because it does not reflect other biomarkers such as PgR, HER-2, and Ki-67. It should be modified by consideration of the prognostic effects of these markers. Although Adjuvant! Online could provide continuous values for survival probability and treatment benefit, clinical decision assumes a dichotomization into low- and high-risk groups. This study used two cutoffs, 88% for ER-positive tumors and 92% for ER-negative tumors, that were decided by consensus among the TRANSBIG consortium members (http://www.breastinternationalgroup.org/). However, Buyse et al. showed poor sensitivity of Adjuvant! Online and improved it by using other prediction models such as the 70-gene signature.6
Although this current study was a retrospective one with a heterogeneous population for adjuvant treatment and short-term median follow-up periods, it showed that Ki-67 has prognostic power regardless of chemotherapy regimens (Fig. 2). To our knowledge, it is the first study to assess the prognostic value of Ki-67 expression combined with the St. Gallen consensus and Adjuvant! Online in early breast cancer.
This study demonstrated that Ki-67 was an independent prognostic factor in early breast cancer and could provide additional prognostic information on the risk stratification with the use of the 2007 St. Gallen consensus and Adjuvant! Online.
References
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–65.
Boyages J, Chua B, Taylor R, et al. Use of the St Gallen classification for patients with node-negative breast cancer may lead to overuse of adjuvant chemotherapy. Br J Surg. 2002;89:789–96.
Iwamoto E, Fukutomi T, Akashi-Tanaka S. Validation and problems of St Gallen recommendations of adjuvant therapy for node-negative invasive breast cancer in Japanese patients. Jpn J Clin Oncol. 2001;31:259–62.
Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–91.
Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–92.
Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–19.
Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–8.
Sun JM, Han W, Im SA, et al. A combination of HER-2 status and the St. Gallen classification provides useful information on prognosis in lymph node-negative breast carcinoma. Cancer. 2004;101:2516–22.
Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Colozza M, Azambuja E, Cardoso F, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–39.
Gerdes J, Li L, Schlueter C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–73.
Cher ML, Chew K, Rosenau W, Carroll PR. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate. 1995;26:87–93.
Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–70.
Caly M, Genin P, Ghuzlan AA, et al. Analysis of correlation between mitotic index, MIB1 score and S-phase fraction as proliferation markers in invasive breast carcinoma Methodological aspects and prognostic value in a series of 257 cases. Anticancer Res. 2004;24:3283–8.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–13.
Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker?. Cancer. 2003;97:1321–31.
Molino A, Micciolo R, Turazza M, et al. Ki-67 immunostaining in 322 primary breast cancers: associations with clinical and pathological variables and prognosis. Int J Cancer. 1997;74:433–7.
Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Billgren AM, Tani E, Liedberg A, et al. Prognostic significance of tumor cell proliferation analyzed in fine needle aspirates from primary breast cancer. Breast Cancer Res Treat. 2002;71:161–70.
Brown RW, Allred CD, Clark GM, et al. Prognostic value of Ki-67 compared to S-phase fraction in axillary node-negative breast cancer. Clin Cancer Res. 1996;2:585–92.
Erdem O, Dursun A, Coskun U, Gunel N. The prognostic value of p53 and c-erbB-2 expression, proliferative activity and angiogenesis in node-negative breast carcinoma. Tumori. 2005;91:46–52.
Rudolph P, Alm P, Heidebrecht HJ, et al. Immunologic proliferation marker Ki-S2 as prognostic indicator for lymph node-negative breast cancer. J Natl Cancer Inst. 1999;91:271–8.
Pietilainen T, Lipponen P, Aaltomaa S, et al. The important prognostic value of Ki-67 expression as determined by image analysis in breast cancer. J Cancer Res Clin Oncol. 1996;122:687–92.
Jacquemier J, Ginestier C, Rougemont J, et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005;65:767–79.
Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9.
van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Malaise EP, Chavaudra N, Tubiana M. The relationship between growth rate, labelling index and histological type of human solid tumours. Eur J Cancer. 1973;9:305–12.
Dean PN, Dolbeare F, Gratzner H, et al. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984;17:427–36.
Quirke P, Dyson JE. Flow cytometry: methodology and applications in pathology. J Pathol. 1986;149:79–87.
Thor AD, Liu S, Moore DH II, Edgerton SM. Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol. 1999;17:470–7.
Lau R, Grimson R, Sansome C, et al. Low levels of cell cycle inhibitor p27kip1 combined with high levels of Ki-67 predict shortened disease-free survival in T1 and T2 invasive breast carcinomas. Int J Oncol. 2001;18:17–23.
Railo M, Lundin J, Haglund C, et al. Ki-67, p53, ER receptors, ploidy and S phase as long-term prognostic factors in T1 node-negative breast cancer. Tumour Biol. 2007;28:45–51.
Spyratos F, Ferrero-Pous M, Trassard M, et al. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94:2151–9.
Volpi A, Nanni O, De Paola F, et al. HER-2 expression and cell proliferation: prognostic markers in patients with node-negative breast cancer. J Clin Oncol. 2003;21:2708–12.
Veronese SM, Gambacorta M, Gottardi O, et al. Proliferation index as a prognostic marker in breast cancer. Cancer. 1993;71:3926–31.
Palazzi M, De Tomasi D, D’Affronto C, et al. Are international guidelines for the prescription of adjuvant treatment for early breast cancer followed in clinical practice? Results of a population-based study on 1547 patients. Tumori. 2002;88:503–6.
Roila F, Ballatori E, Patoia L, et al. Adjuvant systemic therapies in women with breast cancer: an audit of clinical practice in Italy. Ann Oncol. 2003;14:843–8.
Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model Adjuvant! for early breast cancer. J Clin Oncol. 2005;23:2716–25.
Acknowledgment
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ3-PG6-01GN07-0004) and by a grant from the Korean Ministry of Education, Science, and Technology, FPR08A2-080 of the 21C Frontier Functional Proteomics Program.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
S.-Y. Jung and W. Han contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jung, SY., Han, W., Lee, J.W. et al. Ki-67 Expression Gives Additional Prognostic Information on St. Gallen 2007 and Adjuvant! Online Risk Categories in Early Breast Cancer. Ann Surg Oncol 16, 1112–1121 (2009). https://doi.org/10.1245/s10434-009-0334-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0334-7