Abstract
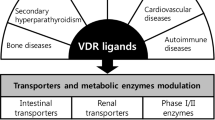
The vitamin D receptor (VDR), in addition to other nuclear receptors, the pregnane X receptor (PXR) and constitutive androstane receptor (CAR), is involved in the regulation of enzymes, transporters and receptors, and therefore intimately affects drug disposition, tissue health, and the handling of endogenous and exogenous compounds. This review examines the role of 1α,25-dihydroxyvitamin D3 or calcitriol, the natural VDR ligand, on activation of the VDR and its crosstalk with other nuclear receptors towards the regulation of enzymes and transporters, notably many of the cytochrome P450s including CYP3A4 and sulfotransferase 2A1 (SULT2A1) as well as cholesterol 7α-hydroxylase (CYP7A1). Moreover, the VDR upregulates the intestinal channel, TRPV6, for calcium absorption, LDL receptor-related protein 1 (LRP1) and receptor for advanced glycation end products (RAGE) in brain for β-amyloid peptide efflux and influx, the sodium phosphate transporters (NaPi), the apical sodium-dependent bile acid transporter (ASBT) and organic solute transporters (OSTα-OSTβ) for bile acid absorption and efflux, respectively, the renal organic anion transporter 3 (OAT3) and several of the ATP-binding cassette protein transporters—the multidrug resistance protein 1 (MDR1) and the multidrug resistance-associated proteins (MRPs). Hence, the role of the VDR is increasingly being recognized for its therapeutic potential and pharmacologic activity, giving rise to drug-drug interactions (DDI). Therapeutically, ligand-activated VDR shows anti-inflammatory effects towards the suppression of inflammatory mediators, improves cognition by upregulating amyloid-beta (Aβ) peptide clearance in brain, and maintains phosphate, calcium, and parathyroid hormone (PTH) balance and kidney function and bone health, demonstrating the crucial roles of the VDR in disease progression and treatment of diseases.





Similar content being viewed by others
Abbreviations
- 1α(OH)D2 :
-
1α-Hydroxyvitamin D2
- 1α(OH)D3 :
-
1α-Hydroxyvitamin D3 or Alfacalcidol®
- 1,24(OH)2D3 :
-
1α,24-Dihydroxyvitamin D3 or Tacalcitol®
- 1,24,25(OH)3D3 :
-
1α,24,25-Trihydroxyvitamin D3
- 1,25(OH)2D2 :
-
1α,25-Dihydroxyvitamin D2
- 1,25(OH)2D3 :
-
1α,25-Dihydroxyvitamin D3 or calcitriol (active VDR ligand)
- 24,25(OH)2D3 :
-
24,25-Dihydroxyvitamin D3
- 25(OH)D3 :
-
25-Hydroxyvitamin D3
- Aβ:
-
β-Amyloid peptide
- ABC:
-
ATP-binding cassette
- ADL:
-
Alzheimer’s disease
- ASBT:
-
Apical sodium-dependent bile acid transporter
- BBB:
-
Blood–brain barrier
- BCRP:
-
Breast cancer resistance protein
- CAR:
-
Constitutive androstane receptor
- CDCA:
-
Chenodeoxycholic acid
- CKD:
-
Chronic kidney disease
- CYP2R1:
-
Cytochrome P450 isoform 2R1, microsomal vitamin D 25-hydroxylase
- CYP3A4:
-
Cytochrome P450 isoform 3A4
- CYP7A1:
-
Cytochrome P450 isoform 7A1, cholesterol 7α-hydroxylase
- CYP24A1:
-
Cytochrome P450 isoform 24A1, 24-hydroxylase
- CYP27A1:
-
Cytochrome P450 isoform 27A1, mitochondrial vitamin D 25-hydroxylase
- CYP27B11:
-
Cytochrome P450 isoform 27B1, mitochondrial 1α-hydroxylase
- DBD:
-
DNA-binding domain
- DBP:
-
Vitamin D-binding protein
- Fgf15:
-
Fibroblast growth factor 15
- FGF19:
-
Fibroblast growth factor 19
- FGF23:
-
Fibroblast growth factor 23
- FGFR4:
-
Fibroblast growth factor receptor 4
- FRα:
-
Folate receptor-α
- FXR:
-
Farnesoid X receptor
- LBD:
-
Ligand-binding domain
- LBP:
-
Ligand-binding pocket
- LCA:
-
Lithocholic acid
- LRH1:
-
Liver receptor homolog 1
- LRP1:
-
LDL receptor-related protein 1
- LXRα:
-
Liver X receptor-α
- MDR1:
-
Multidrug resistance protein 1
- MRP2:
-
Multidrug resistance-associated protein 2
- MRP3:
-
Multidrug resistance-associated protein 3
- MRP4:
-
Multidrug resistance-associated protein 4
- NaPi :
-
Sodium-dependent phosphate cotransporter
- NR:
-
Nuclear receptor
- OAT1:
-
Organic anion transporter 1
- OAT3:
-
Organic anion transporter 3
- OSTα-OSTβ:
-
Organic solute transporter-α-β
- PCFT:
-
Proton-coupled folate transporter
- PEPT1:
-
Oligopeptide transporter 1
- P-gp:
-
P-Glycoprotein
- PPARα:
-
Peroxisome proliferator-activated receptor-α
- PPARγ:
-
Peroxisome proliferator-activated receptor-γ
- PTH:
-
Parathyroid hormone
- PXR:
-
Pregnane X-receptor
- RAGE:
-
Receptor for advanced glycation end products
- RFC:
-
Reduced folate carrier
- RXR:
-
Retinoid X receptor
- SHP:
-
Small heterodimer partner
- SHPT:
-
Secondary hyperparathyroidism
- SLC:
-
Solute carrier
- SULT2A1:
-
Sulfotransferase 2A1
- TRPV5:
-
Transient receptor potential cation channel, subfamily V, member 5
- TRPV6:
-
Transient receptor potential cation channel, subfamily V, member 6
- UGT:
-
UDP glucuronosyltransferase
- VDR:
-
Vitamin D receptor
- VDRE:
-
Vitamin D response element
References
Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. https://doi.org/10.1210/er.2001-0038.
Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66(3):413–9. https://doi.org/10.1124/mol.66.3.
Prakash C, Zuniga B, Song CS, Jiang S, Cropper J, Park S, et al. Nuclear receptors in drug metabolism, drug response and drug interactions. Nucl Receptor Res. 2015;2. doi: https://doi.org/10.11131/2015/101178.
Makishima M, Lu TT, **e W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–6. https://doi.org/10.1126/science.1070477.
Thompson PD, Jurutka PW, Whitfield GK, Myskowski SM, Eichhorst KR, Dominguez CE, et al. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun. 2002;299(5):730–8. https://doi.org/10.1016/s0006-291x(02)02742-0.
Xu Y, Iwanaga K, Zhou C, Cheesman MJ, Farin F, Thummel KE. Selective induction of intestinal CYP3A23 by 1α,25-dihydroxyvitamin D3 in rats. Biochem Pharmacol. 2006;72(3):385–92. https://doi.org/10.1016/j.bcp.2006.04.033.
Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004;65(3):720–9. https://doi.org/10.1124/mol.65.3.720.
Chatterjee B, Echchgadda I, Song CS. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005;400:165–91. https://doi.org/10.1016/S0076-6879(05)00010-8.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–9. https://doi.org/10.1016/0092-8674(95)90199-x.
Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53(3):1023–34. https://doi.org/10.1002/hep.24148.
Olefsky JM. Nuclear receptor minireview series. J Biol Chem. 2001;276(40):36863–4. https://doi.org/10.1074/jbc.R100047200.
Sandgren ME, Bronnegard M, DeLuca HF. Tissue distribution of the 1,25-dihydroxyvitamin D3 receptor in the male rat. Biochem Biophys Res Commun. 1991;181(2):611–6. https://doi.org/10.1016/0006-291x(91)91234-4.
Liesegang A, Singer K, Boos A. Vitamin D receptor amounts across different segments of the gastrointestinal tract in Brown Swiss and Holstein Frisean cows of different age. J Anim Physiol Anim Nutr (Berl). 2008;92(3):316–23. https://doi.org/10.1111/j.1439-0396.2007.00782.x.
Colston K, Hirt M, Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980;107(6):1916–22. https://doi.org/10.1210/endo-107-6-1916.
Chow EC, Quach HP, Vieth R, Pang KS. Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. Am J Physiol Endocrinol Metab. 2013;304(9):E977–89. https://doi.org/10.1152/ajpendo.00489.2012.
Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37(5):1034–42. https://doi.org/10.1053/jhep.2003.50176.
Chow EC, Magomedova L, Quach HP, Patel R, Durk MR, Fan J, et al. Vitamin D receptor activation down-regulates the small heterodimer partner and increases CYP7A1 to lower cholesterol. Gastroenterology. 2014;146(4):1048–59. https://doi.org/10.1053/j.gastro.2013.12.027.
Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135–45. https://doi.org/10.1016/s0891-0618(99)00002-2.
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. https://doi.org/10.1016/j.jchemneu.2004.08.006.
Kamei Y, Kawada T, Fukuwatari T, Ono T, Kato S, Sugimoto E. Cloning and sequencing of the gene encoding the mouse vitamin D receptor. Gene. 1995;152(2):281–2. https://doi.org/10.1016/0378-1119(94)00735-b.
Shaffer PL, McDonnell DP, Gewirth DT. Characterization of transcriptional activation and DNA-binding functions in the hinge region of the vitamin D receptor. Biochemistry. 2005;44(7):2678–85. https://doi.org/10.1021/bi0477182.
Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28. https://doi.org/10.1152/ajprenal.00336.2004.
Hourai S, Fujishima T, Kittaka A, Suhara Y, Takayama H, Rochel N, et al. Probing a water channel near the A-ring of receptor-bound 1α,25-dihydroxyvitamin D3 with selected 2α-substituted analogues. J Med Chem. 2006;49(17):5199–205. https://doi.org/10.1021/jm0604070.
Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF. Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry. 2004;43(14):4101–10. https://doi.org/10.1021/bi036056y.
Feldman D, P JM. Mutations in the vitamin D receptor and hereditary vitamin D-resistant rickets. Bonekey Rep. 2014;3:510. doi: https://doi.org/10.1038/bonekey.2014.5.
Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5(1):173–9. https://doi.org/10.1016/s1097-2765(00)80413-x.
Maestro MA, Molnar F, Carlberg C. Vitamin D and its synthetic analogs. J Med Chem. 2019;62(15):6854–75. https://doi.org/10.1021/acs.jmedchem.9b00208.
Wan LY, Zhang YQ, Chen MD, Du YQ, Liu CB, Wu JF. Relationship between structure and conformational change of the vitamin D receptor ligand binding domain in 1alpha,25-dihydroxyvitamin D3 signaling. Molecules. 2015;20(11):20473–86. https://doi.org/10.3390/molecules201119713.
Yamada S, Yamamoto K. Ligand recognition by vitamin D receptor: total alanine scanning mutational analysis of the residues lining the ligand binding pocket of vitamin D receptor. Curr Top Med Chem. 2006;6(12):1255–65. https://doi.org/10.2174/156802606777864881.
Brumbaugh PF, Haussler MR. Specific binding of 1α,25-dihydroxycholecalciferol to nuclear components of chick intestine. J Biol Chem. 1975;250(4):1588–94.
Walters MR, Hunziker W, Norman AW. Unoccupied 1,25-dihydroxyvitamin D3 receptors. Nuclear/cytosol ratio depends on ionic strength. J Biol Chem. 1980;255(14):6799–805.
Taketani Y, Segawa H, Chikamori M, Morita K, Tanaka K, Kido S, et al. Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J Biol Chem. 1998;273(23):14575–81. doi: https://doi.org/10.1074/jbc.273.23.14575.
Dawson PA, Markovich D. Transcriptional regulation of the sodium-sulfate cotransporter NaSi-1 gene. Cell Biochem Biophys. 2002;36(2–3):175–82. https://doi.org/10.1385/CBB:36:2-3:175.
den Dekker E, Hoenderop JG, Nilius B, Bindels RJ. The epithelial calcium channels, TRPV5 & TRPV6: from identification towards regulation. Cell Calcium. 2003;33(5–6):497–507. https://doi.org/10.1016/s0143-4160(03)00065-4.
Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–59. https://doi.org/10.1016/j.beem.2011.05.010.
Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Watkins PB. Induction of CYP3A4 by 1α,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab Dispos. 2001;29(11):1446–53.
Pfrunder A, Gutmann H, Beglinger C, Drewe J. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol. 2003;55(1):59–66. https://doi.org/10.1111/j.2042-7158.2003.tb02434.x.
Aiba T, Susa M, Fukumori S, Hashimoto Y. The effects of culture conditions on CYP3A4 and MDR1 mRNA induction by 1α,25-dihydroxyvitamin D3 in human intestinal cell lines, Caco-2 and LS180. Drug Metab Pharmacokinet. 2005;20(4):268–74. https://doi.org/10.2133/dmpk.20.268.
Saeki M, Kurose K, Tohkin M, Hasegawa R. Identification of the functional vitamin D response elements in the human MDR1 gene. Biochem Pharmacol. 2008;76(4):531–42. https://doi.org/10.1016/j.bcp.2008.05.030.
Fan J, Liu S, Du Y, Morrison J, Shipman R, Pang KS. Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1α,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J Pharmacol Exp Ther. 2009;330(2):389–402. https://doi.org/10.1124/jpet.108.149815.
** CH, Kerner SA, Hong MH, Pike JW. Transcriptional activation and dimerization functions in the human vitamin D receptor. Mol Endocrinol. 1996;10(8):945–57. https://doi.org/10.1210/mend.10.8.8843411.
Colnot S, Lambert M, Blin C, Thomasset M, Perret C. Identification of DNA sequences that bind retinoid X receptor-1,25(OH)2D3-receptor heterodimers with high affinity. Mol Cell Endocrinol. 1995;113(1):89–98. https://doi.org/10.1016/0303-7207(95)03618-h.
Malloy PJ, Xu R, Peng L, Clark PA, Feldman D. A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol. 2002;16(11):2538–46. https://doi.org/10.1210/me.2002-0152.
Zhang J, Chalmers MJ, Stayrook KR, Burris LL, Wang Y, Busby SA, et al. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat Struct Mol Biol. 2011;18(5):556–63. https://doi.org/10.1038/nsmb.2046.
Chen X, Chen F, Liu S, Glaeser H, Dawson PA, Hofmann AF, et al. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1α,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol Pharmacol. 2006;69(6):1913–23. https://doi.org/10.1124/mol.105.020792.
Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7(4):349–65. https://doi.org/10.2174/138920006776873526.
Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115(1):177–86. https://doi.org/10.1172/JCI21867.
Moreau A, Maurel P, Vilarem MJ, Pascussi JM. Constitutive androstane receptor-vitamin D receptor crosstalk: consequence on CYP24 gene expression. Biochem Biophys Res Commun. 2007;360(1):76–82. https://doi.org/10.1016/j.bbrc.2007.06.003.
Honjo Y, Sasaki S, Kobayashi Y, Misawa H, Nakamura H. 1,25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. J Endocrinol. 2006;188(3):635–43. https://doi.org/10.1677/joe.1.06105.
Jiang W, Miyamoto T, Kakizawa T, Nishio SI, Oiwa A, Takeda T, et al. Inhibition of LXRα signaling by vitamin D receptor: possible role of VDR in bile acid synthesis. Biochem Biophys Res Commun. 2006;351(1):176–84. https://doi.org/10.1016/j.bbrc.2006.10.027.
Chow EC, Maeng HJ, Liu S, Khan AA, Groothuis GM, Pang KS. 1α,25-Dihydroxyvitamin D3 triggered vitamin D receptor and farnesoid X receptor-like effects in rat intestine and liver in vivo. Biopharm Drug Dispos. 2009;30(8):457–75. https://doi.org/10.1002/bdd.682.
Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285(19):14486–94. https://doi.org/10.1074/jbc.M110.116004.
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26. https://doi.org/10.1016/s1097-2765(00)00051-4.
Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25. https://doi.org/10.1016/j.cmet.2005.09.001.
Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278(22):19909–16. https://doi.org/10.1074/jbc.M207903200.
Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, et al. The heteromeric organic solute transporter α-β, Ostα-Ostβ, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280(8):6960–8. https://doi.org/10.1074/jbc.M412752200.
Li H, Chen F, Shang Q, Pan L, Shneider BL, Chiang JY, et al. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am J Physiol Gastrointest Liver Physiol. 2005;288(1):G60–6. https://doi.org/10.1152/ajpgi.00170.2004.
Sertznig P, Dunlop T, Seifert M, Tilgen W, Reichrath J. Cross-talk between vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling in melanoma cells. Anticancer Res. 2009;29(9):3647–58.
Sertznig P, Seifert M, Tilgen W, Reichrath J. Activation of vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling pathways through 1,25(OH)2D3 in melanoma cell lines and other skin-derived cell lines. Dermatoendocrinol. 2009;1(4):232–8. https://doi.org/10.4161/derm.1.4.9629.
Alimirah F, Peng X, Yuan L, Mehta RR, von Knethen A, Choubey D, et al. Crosstalk between the peroxisome proliferator-activated receptor γ (PPARγ) and the vitamin D receptor (VDR) in human breast cancer cells: PPARγ binds to VDR and inhibits 1α,25-dihydroxyvitamin D3 mediated transactivation. Exp Cell Res. 2012;318(19):2490–7. https://doi.org/10.1016/j.yexcr.2012.07.020.
Quach HP, Noh K, Hoi SY, Bruinsma A, Groothuis GMM, Li AP, et al. Alterations in gene expression in vitamin D-deficiency: down-regulation of liver Cyp7a1 and renal Oat3 in mice. Biopharm Drug Dispos. 2018;39(2):99–115. https://doi.org/10.1002/bdd.2118.
Kim YC, Kim IB, Noh CK, Quach HP, Yoon IS, Chow ECY, et al. Effects of 1α,25-dihydroxyvitamin D3, the natural vitamin D receptor ligand, on the pharmacokinetics of cefdinir and cefadroxil, organic anion transporter substrates, in rat. J Pharm Sci. 2014;103(11):3793–805. https://doi.org/10.1002/jps.24195.
Simboli-Campbell M, Franks DJ, Welsh J. 1,25(OH)2D3 increases membrane associated protein kinase C in MDBK cells. Cell Signal. 1992;4(1):99–109. https://doi.org/10.1016/0898-6568(92)90011-v.
Simboli-Campbell M, Gagnon A, Franks DJ, Welsh J. 1,25-Dihydroxyvitamin D3 translocates protein kinase C beta to nucleus and enhances plasma membrane association of protein kinase C alpha in renal epithelial cells. J Biol Chem. 1994;269(5):3257–64.
Takeda M, Sekine T, Endou H. Regulation by protein kinase C of organic anion transport driven by rat organic anion transporter 3 (rOAT3). Life Sci. 2000;67(9):1087–93. https://doi.org/10.1016/s0024-3205(00)00694-9.
Redling S, Pfaff IL, Leitges M, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta I, beta II, delta, and epsilon in mouse kidney. Am J Physiol Renal Physiol. 2004;287(2):F289–98. https://doi.org/10.1152/ajprenal.00273.2003.
Pfaff IL, Wagner HJ, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol. 1999;10(9):1861–73. https://doi.org/10.1681/ASN.V1091861.
Chow EC, Durk MR, Maeng HJ, Pang KS. Comparative effects of 1α-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on transporters and enzymes in fxr(+/+) and fxr(-/-) mice. Biopharm Drug Dispos. 2013;34(7):402–16. https://doi.org/10.1002/bdd.1856.
McCarthy TC, Li X, Sinal CJ. Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem. 2005;280(24):23232–42. https://doi.org/10.1074/jbc.M411520200.
Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G476–85. https://doi.org/10.1152/ajpgi.00430.2005.
Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40(1):149–56. https://doi.org/10.1002/hep.20295.
Xu S, Sun AQ, Suchy FJ. A novel RARalpha/CAR-mediated mechanism for regulation of human organic solute transporter-beta gene expression. Am J Physiol Gastrointest Liver Physiol. 2014;306(2):G154–62. https://doi.org/10.1152/ajpgi.00138.2013.
Park S, Cheng SL, Cui JY. Characterizing drug-metabolizing enzymes and transporters that are bona fide CAR-target genes in mouse intestine. Acta Pharm Sin B. 2016;6(5):475–91. https://doi.org/10.1016/j.apsb.2016.07.004.
Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121(1):140–7. https://doi.org/10.1053/gast.2001.25503.
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276(31):28857–65. https://doi.org/10.1074/jbc.M011610200.
Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1124–30. https://doi.org/10.1152/ajpgi.00539.2005.
Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39(4):480–8. https://doi.org/10.1016/s0168-8278(03)00228-9.
Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42(2):420–30. https://doi.org/10.1002/hep.20784.
Brommage R, DeLuca HF. Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr Rev. 1985;6(4):491–511. https://doi.org/10.1210/edrv-6-4-491.
Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–231. https://doi.org/10.1152/physrev.1998.78.4.1193.
Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D binding protein: a historic overview. Front Endocrinol (Lausanne). 2019;10:910. https://doi.org/10.3389/fendo.2019.00910.
Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin). Endocr Rev. 1989;10(3):294–307. https://doi.org/10.1210/edrv-10-3-294.
Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28(3):252–6. https://doi.org/10.1080/07315724.2009.10719779.
Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–34. https://doi.org/10.2741/1514.
Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxylase. J Biol Chem. 2003;278(39):38084–93. https://doi.org/10.1074/jbc.M307028200.
DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2(3):224–36.
Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989;28(4):1763–9. https://doi.org/10.1021/bi00430a051.
Noh K, Yang QJ, Sekhon L, Quach HP, Chow ECY, Pang KS. Noteworthy idiosyncrasies of 1α,25-dihydroxyvitamin D3 kinetics for extrapolation from mouse to man: commentary. Biopharm Drug Dispos. 2020;41(3):126–48. https://doi.org/10.1002/bdd.2223.
Meyer MB, Pike JW. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J Steroid Biochem Mol Biol. 2020;196: 105500. https://doi.org/10.1016/j.jsbmb.2019.105500.
Gao C, Bergagnini-Kolev MC, Liao MZ, Wang Z, Wong T, Calamia JC, et al. Simultaneous quantification of 25-hydroxyvitamin D3–3-sulfate and 25-hydroxyvitamin D3–3-glucuronide in human serum and plasma using liquid chromatography-tandem mass spectrometry coupled with DAPTAD-derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:158–65. https://doi.org/10.1016/j.jchromb.2017.06.017.
Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013;136:54–8. https://doi.org/10.1016/j.jsbmb.2012.09.012.
Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, et al. Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility, and interindividual variability. Endocrinology. 2014;155(6):2052–63. https://doi.org/10.1210/en.2013-2013.
Cooke NE, Walgate J, Haddad JG, Jr. Human serum binding protein for vitamin D and its metabolites. II. Specific, high affinity association with a protein in nucleated tissue. J Biol Chem. 1979;254(13):5965–71.
Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969–75. https://doi.org/10.1210/jcem-61-5-969.
Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest. 1986;78(3):748–52. https://doi.org/10.1172/JCI112636.
Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103(2):239–51. https://doi.org/10.1172/JCI5244.
Yamane T, Takeuchi K, Yamamoto Y, Li YH, Fujiwara M, Nishi K, et al. Legumain from bovine kidney: its purification, molecular cloning, immunohistochemical localization and degradation of annexin II and vitamin D-binding protein. Biochim Biophys Acta. 2002;1596(1):108–20. https://doi.org/10.1016/s0167-4838(02)00209-1.
Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228(5273):764–6. https://doi.org/10.1038/228764a0.
Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, et al. Cloning of human 25-hydroxyvitamin D-1α-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11(13):1961–70. https://doi.org/10.1210/mend.11.13.0035.
Anderson PH, Hendrix I, Sawyer RK, Zarrinkalam R, Manavis J, Sarvestani GT, et al. Co-expression of CYP27B1 enzyme with the 1.5kb CYP27B1 promoter-luciferase transgene in the mouse. Mol Cell Endocrinol. 2008;285(1–2):1–9. doi: https://doi.org/10.1016/j.mce.2007.12.018.
Hollis BW. Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int. 1996;58(1):4–5. https://doi.org/10.1007/BF02509538.
Vieth R. What is the optimal vitamin D status for health? Prog Biophys Mol Biol. 2006;92(1):26–32. https://doi.org/10.1016/j.pbiomolbio.2006.02.003.
Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142(3):498–507. doi: https://doi.org/10.3945/jn.111.151977.
van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25(4):671–80. https://doi.org/10.1016/j.beem.2011.06.007.
Cianferotti L, Marcocci C. Subclinical vitamin D deficiency. Best Pract Res Clin Endocrinol Metab. 2012;26(4):523–37. https://doi.org/10.1016/j.beem.2011.12.007.
Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–6. https://doi.org/10.1007/s00198-005-1867-7.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. https://doi.org/10.1210/jc.2011-0385.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. https://doi.org/10.1056/NEJMra070553.
Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 Suppl 5):S3–S19. https://doi.org/10.1053/ajkd.2001.28111.
Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Mol Aspects Med. 2008;29(6):433–52. https://doi.org/10.1016/j.mam.2008.04.001.
Sjoden G, Smith C, Lindgren U, DeLuca HF. 1α-Hydroxyvitamin D2 is less toxic than 1α-hydroxyvitamin D3 in the rat. Proc Soc Exp Biol Med. 1985;178(3):432–6. https://doi.org/10.3181/00379727-178-42028.
Nehring JA, Zierold C, DeLuca HF. Lithocholic acid can carry out in vivo functions of vitamin D. Proc Natl Acad Sci U S A. 2007;104(24):10006–9. https://doi.org/10.1073/pnas.0703512104.
Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–72. https://doi.org/10.1172/JCI29449.
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. https://doi.org/10.1161/CIRCULATIONAHA.107.706127.
Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69(1):33–43. https://doi.org/10.1038/sj.ki.5000045.
Bhattarai HK, Shrestha S, Rokka K, Shakya R. Vitamin D, calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J Osteoporos. 2020;2020:9324505. https://doi.org/10.1155/2020/9324505.
Silver J, Levi R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int Suppl. 2005;95:S8-12. https://doi.org/10.1111/j.1523-1755.2005.09501.x.
Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74(6):2136–43. https://doi.org/10.1172/JCI111639.
Takeda E, Taketani Y, Sawada N, Sato T, Yamamoto H. The regulation and function of phosphate in the human body. BioFactors. 2004;21(1–4):345–55. https://doi.org/10.1002/biof.552210167.
Goyal R, Jialal I. Hyperphosphatemia. StatPearls. Treasure Island (FL)2021.
Goretti Penido M, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27(11):2039–48. https://doi.org/10.1007/s00467-012-2175-z.
Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. https://doi.org/10.1146/annurev.med.051308.111339.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282–91. https://doi.org/10.1152/ajprenal.90742.2008.
Silver J, Naveh-Many T. FGF23 and the parathyroid. Adv Exp Med Biol. 2012;728:92–9. https://doi.org/10.1007/978-1-4614-0887-1_6.
Yang QJ, Bukuroshi P, Quach HP, Chow ECY, Pang KS. Highlighting vitamin D receptor-targeted activities of 1α,25-dihydroxyvitamin D3 in mice via physiologically based pharmacokinetic-pharmacodynamic modeling. Drug Metab Dispos. 2018;46(1):75–87. https://doi.org/10.1124/dmd.117.077271.
Enomoto H, Hendy GN, Andrews GK, Clemens TL. Regulation of avian calbindin-D28K gene expression in primary chick kidney cells: importance of posttranscriptional mechanisms and calcium ion concentration. Endocrinology. 1992;130(6):3467–74. https://doi.org/10.1210/endo.130.6.1375904.
Murer H, Hernando N, Forster I, Biber J. Molecular aspects in the regulation of renal inorganic phosphate reabsorption: the type IIa sodium/inorganic phosphate co-transporter as the key player. Curr Opin Nephrol Hypertens. 2001;10(5):555–61. https://doi.org/10.1097/00041552-200109000-00002.
Hildmann B, Storelli C, Danisi G, Murer H. Regulation of Na+-Pi cotransport by 1,25-dihydroxyvitamin D3 in rabbit duodenal brush-border membrane. Am J Physiol. 1982;242(5):G533–9. https://doi.org/10.1152/ajpgi.1982.242.5.G533.
Hamano T, Nakano C, Obi Y, Fujii N, Matsui I, Tomida K, et al. Fibroblast growth factor 23 and 25-hydroxyvitamin D levels are associated with estimated glomerular filtration rate decline. Kidney Int Suppl (2011). 2013;3(5):469–75. doi: https://doi.org/10.1038/kisup.2013.97.
Koizumi M, Komaba H, Fukagawa M. Parathyroid function in chronic kidney disease: role of FGF23-Klotho axis. Contrib Nephrol. 2013;180:110–23. https://doi.org/10.1159/000346791.
Tani T, Orimo H, Shimizu A, Tsuruoka S. Development of a novel chronic kidney disease mouse model to evaluate the progression of hyperphosphatemia and associated mineral bone disease. Sci Rep. 2017;7(1):2233. https://doi.org/10.1038/s41598-017-02351-6.
Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. https://doi.org/10.1159/000346778.
Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67(2):115–27. https://doi.org/10.1017/S0029665108006964.
Voutsadakis IA. Vitamin D baseline levels at diagnosis of breast cancer: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2021;14(1):16–26. https://doi.org/10.1016/j.hemonc.2020.08.005.
Negri M, Gentile A, de Angelis C, Monto T, Patalano R, Colao A, et al. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients. 2020;12(6). doi: https://doi.org/10.3390/nu12061798.
Henner WD, Beer TM. A new formulation of calcitriol (DN-101) for high-dose pulse administration in prostate cancer therapy. Rev Urol. 2003;5(Suppl 3):S38-44.
Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol. 2003;21(1):123–8. https://doi.org/10.1200/jco.2003.05.117.
Muindi JR, Potter DM, Peng Y, Johnson CS, Trump DL. Pharmacokinetics of liquid calcitriol formulation in advanced solid tumor patients: comparison with caplet formulation. Cancer Chemother Pharmacol. 2005;56(5):492–6. https://doi.org/10.1007/s00280-005-1015-2.
Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216–23. https://doi.org/10.1158/1078-0432.CCR-06-1165.
Barrea L, Savanelli MC, Di Somma C, Napolitano M, Megna M, Colao A, et al. Vitamin D and its role in psoriasis: an overview of the dermatologist and nutritionist. Rev Endocr Metab Disord. 2017;18(2):195–205. https://doi.org/10.1007/s11154-017-9411-6.
Durk MR, Han K, Chow EC, Ahrens R, Henderson JT, Fraser PE, et al. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-beta accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neurosci. 2014;34(21):7091–101. https://doi.org/10.1523/JNEUROSCI.2711-13.2014.
Chow EC, Sun H, Khan AA, Groothuis GM, Pang KS. Effects of 1α,25-dihydroxyvitamin D3 on transporters and enzymes of the rat intestine and kidney in vivo. Biopharm Drug Dispos. 2010;31(1):91–108. https://doi.org/10.1002/bdd.694.
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie.” Drug Metab Dispos. 2006;34(5):880–6. https://doi.org/10.1124/dmd.105.008672.
Galetin A, Gertz M, Houston JB. Potential role of intestinal first-pass metabolism in the prediction of drug-drug interactions. Expert Opin Drug Metab Toxicol. 2008;4(7):909–22. https://doi.org/10.1517/17425255.4.7.909.
Khan AA, Chow EC, van Loenen-Weemaes AM, Porte RJ, Pang KS, Groothuis GM. Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur J Pharm Sci. 2009;37(2):115–25. https://doi.org/10.1016/j.ejps.2009.01.006.
Zierold C, Mings JA, Deluca HF. 19nor-1,25-Dihydroxyvitamin D2 specifically induces CYP3A9 in rat intestine more strongly than 1,25-dihydroxyvitamin D3 in vivo and in vitro. Mol Pharmacol. 2006;69(5):1740–7. https://doi.org/10.1124/mol.105.019851.
Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol. 2007;218(1):37–44. https://doi.org/10.1016/j.taap.2006.10.005.
Wang K, Chen S, **e W, Wan YJ. Retinoids induce cytochrome P450 3A4 through RXR/VDR-mediated pathway. Biochem Pharmacol. 2008;75(11):2204–13. https://doi.org/10.1016/j.bcp.2008.02.030.
Chow EC, Sondervan M, ** C, Groothuis GM, Pang KS. Comparative effects of doxercalciferol (1α-hydroxyvitamin D2) versus calcitriol (1α,25-dihydroxyvitamin D3) on the expression of transporters and enzymes in the rat in vivo. J Pharm Sci. 2011;100(4):1594–604. https://doi.org/10.1002/jps.22366.
Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Lown KS, Watkins PB. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1α,25-dihydroxyvitamin D3. Mol Pharmacol. 1997;51(5):741–54. https://doi.org/10.1124/mol.51.5.741.
Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1α,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60(6):1399–406. https://doi.org/10.1124/mol.60.6.1399.
Adachi R, Honma Y, Masuno H, Kawana K, Shimomura I, Yamada S, et al. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J Lipid Res. 2005;46(1):46–57. https://doi.org/10.1194/jlr.M400294-JLR200.
Jurutka PW, Thompson PD, Whitfield GK, Eichhorst KR, Hall N, Dominguez CE, et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D3 and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J Cell Biochem. 2005;94(5):917–43. https://doi.org/10.1002/jcb.20359.
Matsubara T, Yoshinari K, Aoyama K, Sugawara M, Sekiya Y, Nagata K, et al. Role of vitamin D receptor in the lithocholic acid-mediated CYP3A induction in vitro and in vivo. Drug Metab Dispos. 2008;36(10):2058–63. https://doi.org/10.1124/dmd.108.021501.
Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277(28):25125–32. https://doi.org/10.1074/jbc.M201323200.
Nishida S, Ozeki J, Makishima M. Modulation of bile acid metabolism by 1α-hydroxyvitamin D3 administration in mice. Drug Metab Dispos. 2009;37(10):2037–44. https://doi.org/10.1124/dmd.109.027334.
Ogura M, Nishida S, Ishizawa M, Sakurai K, Shimizu M, Matsuo S, et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J Pharmacol Exp Ther. 2009;328(2):564–70. https://doi.org/10.1124/jpet.108.145987.
Khan AA, Chow EC, Porte RJ, Pang KS, Groothuis GM. The role of lithocholic acid in the regulation of bile acid detoxication, synthesis, and transport proteins in rat and human intestine and liver slices. Toxicol In Vitro. 2011;25(1):80–90. https://doi.org/10.1016/j.tiv.2010.09.011.
Song CS, Echchgadda I, Seo YK, Oh T, Kim S, Kim SA, et al. An essential role of the CAAT/enhancer binding protein-alpha in the vitamin D-induced expression of the human steroid/bile acid-sulfotransferase (SULT2A1). Mol Endocrinol. 2006;20(4):795–808. https://doi.org/10.1210/me.2005-0428.
Seo YK, Chung YT, Kim S, Echchgadda I, Song CS, Chatterjee B. Xenobiotic- and vitamin D-responsive induction of the steroid/bile acid-sulfotransferase Sult2A1 in young and old mice: the role of a gene enhancer in the liver chromatin. Gene. 2007;386(1–2):218–23. https://doi.org/10.1016/j.gene.2006.10.006.
Eloranta JJ, Zair ZM, Hiller C, Hausler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol. 2009;76(5):1062–71. https://doi.org/10.1124/mol.109.055392.
Alam C, Aufreiter S, Georgiou CJ, Hoque MT, Finnell RH, O’Connor DL, et al. Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci U S A. 2019;116(35):17531–40. https://doi.org/10.1073/pnas.1907077116.
Brandsch C, Zibolka J, Frommhagen M, Lehmann U, Dierkes J, Kuhne H, et al. Vitamin D is not linked to folate status and mRNA expression of intestinal proton-coupled folate transporter. Eur J Nutr. 2014;53(4):1115–22. https://doi.org/10.1007/s00394-013-0614-7.
Alam C, Hoque MT, Finnell RH, Goldman ID, Bendayan R. Regulation of reduced folate carrier (RFC) by vitamin D receptor at the blood-brain barrier. Mol Pharm. 2017;14(11):3848–58. https://doi.org/10.1021/acs.molpharmaceut.7b00572.
Miao Q, Liu Q, Wang C, Meng Q, Guo X, Sun H, et al. Inhibitory effect of 1α,25-dihydroxyvitamin D3 on excretion of JBP485 via organic anion transporters in rats. Eur J Pharm Sci. 2013;48(1–2):351–9. https://doi.org/10.1016/j.ejps.2012.11.008.
Khan AA, Chow EC, Porte RJ, Pang KS, Groothuis GM. Expression and regulation of the bile acid transporter, OSTα-OSTβ in rat and human intestine and liver. Biopharm Drug Dispos. 2009;30(5):241–58. https://doi.org/10.1002/bdd.663.
Maeng HJ, Durk MR, Chow EC, Ghoneim R, Pang KS. 1α,25-Dihydroxyvitamin D3 on intestinal transporter function: studies with the rat everted intestinal sac. Biopharm Drug Dispos. 2011;32(2):112–25. https://doi.org/10.1002/bdd.742.
Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284(7):4267–74. https://doi.org/10.1074/jbc.M807665200.
Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter. OSTα-OSTβ. J Biol Chem. 2003;278(30):27473–82. https://doi.org/10.1074/jbc.M301106200.
Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20(6):1447–61. https://doi.org/10.1210/me.2006-0031.
Patel P, Shah J. Role of vitamin D in amyloid clearance via LRP-1 upregulation in Alzheimer’s disease: a potential therapeutic target? J Chem Neuroanat. 2017;85:36–42. https://doi.org/10.1016/j.jchemneu.2017.06.007.
Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35(11 Suppl 1):2628–31. https://doi.org/10.1161/01.STR.0000143452.85382.d1.
Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. https://doi.org/10.1016/j.neuroscience.2016.01.041.
Durk MR, Chan GN, Campos CR, Peart JC, Chow EC, Lee E, et al. 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem. 2012;123(6):944–53. https://doi.org/10.1111/jnc.12041.
Durk MR, Fan J, Sun H, Yang Y, Pang H, Pang KS, et al. Vitamin D receptor activation induces P-glycoprotein and increases brain efflux of quinidine: an intracerebral microdialysis study in conscious rats. Pharm Res. 2015;32(3):1128–40. https://doi.org/10.1007/s11095-014-1524-y.
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455(1):152–62. https://doi.org/10.1016/0005-2736(76)90160-7.
Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34(1–2):47–54. https://doi.org/10.1081/dmr-120001389.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. https://doi.org/10.1073/pnas.84.21.7735.
Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. https://doi.org/10.1124/pr.107.07109.
Tachibana S, Yoshinari K, Chikada T, Toriyabe T, Nagata K, Yamazoe Y. Involvement of vitamin D receptor in the intestinal induction of human ABCB1. Drug Metab Dispos. 2009;37(8):1604–10. https://doi.org/10.1124/dmd.109.027219.
Chow EC, Durk MR, Cummins CL, Pang KS. 1α,25-Dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(-/-) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther. 2011;337(3):846–59. https://doi.org/10.1124/jpet.111.179101.
Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. 1α,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1–40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS. 2011;8:20. https://doi.org/10.1186/2045-8118-8-20.
Schinkel AH. The roles of P-glycoprotein and MRP1 in the blood-brain and blood-cerebrospinal fluid barriers. Adv Exp Med Biol. 2001;500:365–72. https://doi.org/10.1007/978-1-4615-0667-6_60.
Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461(2):377–94. https://doi.org/10.1016/s0005-2736(99)00169-8.
Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;201:299–323. https://doi.org/10.1007/978-3-642-14541-4_8.
Englund G, Rorsman F, Ronnblom A, Karlbom U, Lazorova L, Grasjo J, et al. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29(3–4):269–77. https://doi.org/10.1016/j.ejps.2006.04.010.
Rost D, Mahner S, Sugiyama Y, Stremmel W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282(4):G720–6. https://doi.org/10.1152/ajpgi.00318.2001.
Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35(8):1333–40. https://doi.org/10.1124/dmd.107.014902.
van Aubel R, Smeets PHE, Peters JGP, Bindels RJM, Russel FGM. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13(3):595–603. https://doi.org/10.1681/ASN.V133595.
Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129(2):349–60. https://doi.org/10.1016/j.neuroscience.2004.07.051.
Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5(9):1048–51. https://doi.org/10.1038/12487.
Mizuno N, Takahashi T, Kusuhara H, Schuetz JD, Niwa T, Sugiyama Y. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one). Drug Metab Dispos. 2007;35(11):2045–52. https://doi.org/10.1124/dmd.107.016352.
Lai L, Tan TM. Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J. 2002;361(Pt 3):497–503. https://doi.org/10.1042/0264-6021:3610497.
Maeng HJ, Chapy H, Zaman S, Pang KS. Effects of 1α,25-dihydroxyvitamin D3 on transport and metabolism of adefovir dipivoxil and its metabolites in Caco-2 cells. Eur J Pharm Sci. 2012;46(3):149–66. https://doi.org/10.1016/j.ejps.2012.02.018.
Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, et al. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281(33):23812–23. https://doi.org/10.1074/jbc.M604890200.
Gorczyca L, Aleksunes LM. Transcription factor-mediated regulation of the BCRP/ABCG2 efflux transporter: a review across tissues and species. Expert Opin Drug Metab Toxicol. 2020;16(3):239–53. https://doi.org/10.1080/17425255.2020.1732348.
Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5(4):797–808. https://doi.org/10.1158/1535-7163.MCT-05-0539.
Ma D, Zhang RN, Wen Y, Yin WN, Bai D, Zheng GY, et al. 1,25(OH)2D3-induced interaction of vitamin D receptor with p50 subunit of NF-kappaB suppresses the interaction between KLF5 and p50, contributing to inhibition of LPS-induced macrophage proliferation. Biochem Biophys Res Commun. 2017;482(2):366–74. https://doi.org/10.1016/j.bbrc.2016.11.069.
Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE. 2011;6(3): e17553. https://doi.org/10.1371/journal.pone.0017553.
Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–8. https://doi.org/10.1212/WNL.0000000000000755.
Keeney JTR, Forster S, Sultana R, Brewer LD, Latimer CS, Cai J, et al. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med. 2013;65:324–34. https://doi.org/10.1016/j.freeradbiomed.2013.07.019.
Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205–11. https://doi.org/10.1093/gerona/gls107.
Laczmanski L, Jakubik M, Bednarek-Tupikowska G, Rymaszewska J, Sloka N, Lwow F. Vitamin D receptor gene polymorphisms in Alzheimer’s disease patients. Exp Gerontol. 2015;69:142–7. https://doi.org/10.1016/j.exger.2015.06.012.
Lee YH, Kim JH, Song GG. Vitamin D receptor polymorphisms and susceptibility to Parkinson’s disease and Alzheimer’s disease: a meta-analysis. Neurol Sci. 2014;35(12):1947–53. https://doi.org/10.1007/s10072-014-1868-4.
Eleftheriadis T, Antoniadi G, Liakopoulos V, Antoniadis N, Stefanidis I, Galaktidou G. Vitamin D receptor activators and response to injury in kidney diseases. J Nephrol. 2010;23(5):514–24.
Zhuang SH, Burnstein KL. Antiproliferative effect of 1α,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology. 1998;139(3):1197–207. https://doi.org/10.1210/endo.139.3.5770.
Muindi JR, Peng Y, Potter DM, Hershberger PA, Tauch JS, Capozzoli MJ, et al. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther. 2002;72(6):648–59. https://doi.org/10.1067/mcp.2002.129305.
Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73(12):1355–63. https://doi.org/10.1038/ki.2008.35.
Moorthi RN, Kandula P, Moe SM. Optimal vitamin D, calcitriol, and vitamin D analog replacement in chronic kidney disease: to D or not to D: that is the question. Curr Opin Nephrol Hypertens. 2011;20(4):354–9. https://doi.org/10.1097/MNH.0b013e3283470450.
Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, et al. Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int. 1998;54(3):907–14. https://doi.org/10.1046/j.1523-1755.1998.00045.x.
Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2):263–76. https://doi.org/10.1053/j.ajkd.2005.10.007.
Greenbaum LA, Benador N, Goldstein SL, Paredes A, Melnick JZ, Mattingly S, et al. Intravenous paricalcitol for treatment of secondary hyperparathyroidism in children on hemodialysis. Am J Kidney Dis. 2007;49(6):814–23. https://doi.org/10.1053/j.ajkd.2007.03.008.
Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):299–306. https://doi.org/10.2215/CJN.07131009.
Dennis VC, Albertson GL. Doxercalciferol treatment of secondary hyperparathyroidism. Ann Pharmacother. 2006;40(11):1955–65. https://doi.org/10.1345/aph.1G523.
Noonan W, Koch K, Nakane M, Ma J, Dixon D, Bolin A, et al. Differential effects of vitamin D receptor activators on aortic calcification and pulse wave velocity in uraemic rats. Nephrol Dial Transplant. 2008;23(12):3824–30. https://doi.org/10.1093/ndt/gfn375.
Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72(6):709–15. https://doi.org/10.1038/sj.ki.5002406.
Acknowledgements
KSP acknowledges the support of NSERC and CIHR.
Author information
Authors and Affiliations
Contributions
Wrote or contributed to the writing of the manuscript: KN, HPQ, ECYC, GMMG, RGT, and KSP.
Contributed data in manuscripts: HPQ, ECYC, KN, GMMG, RGT, and KSP
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noh, K., Chow, E.C.Y., Quach, H.P. et al. Significance of the Vitamin D Receptor on Crosstalk with Nuclear Receptors and Regulation of Enzymes and Transporters. AAPS J 24, 71 (2022). https://doi.org/10.1208/s12248-022-00719-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00719-9