Abstract
Breast milk prebiotic oligosaccharides are believed to promote enteral tolerance. Many mothers delivering preterm are unable to provide sufficient milk. We conducted a multicenter, randomized, controlled trial comparing preterm formula containing 0.8 g/100 mL short-chain galacto-oligosaccharides/long-chain fructo-oligosaccharides in a 9:1 ratio and an otherwise identical formula, using formula only to augment insufficient maternal milk volume. Infants were randomized within 24 h of birth. The primary outcome (PO) was time to establish a total milk intake of 150 mL/kg/d PO and the principal secondary outcome (PSO) was proportion of time between birth and 28 d/discharge that a total milk intake of ≥150 mL/kg/d was tolerated. Other secondary outcomes included growth, fecal characteristics, gastrointestinal signs, necrotizing enterocolitis, and bloodstream infection. Outcomes were compared adjusted for prespecified covariates. We recruited 160 infants appropriately grown for GA <33 wk. There were no significant differences in PO or PSOs. After covariate adjustment, we showed significant benefit from trial formula in PSO with increasing infant immaturity (2.9% improved tolerance for a baby born at 28-wk gestation and 9.9% at 26-wk gestation; p < 0.001) but decreased or no benefit in babies >31-wk gestation. Prebiotic supplementation appears safe and may benefit enteral tolerance in the most immature infants.
Similar content being viewed by others
Main
Nutrition is of crucial importance to the extremely preterm infant. Enteral nutrition is in general preferred over parenteral as the latter has several adverse effects. The tolerance of preterm infants to enteral nutrition is limited by several factors and improved by colonization with probiotic bacteria (1). Bacterial colonization occurs rapidly after delivery and is heavily influenced by infant diet. In the breast-fed baby, the intestinal flora is dominated by bifidobacteria and lactobacilli. Human milk, unlike bovine milk, contains ∼10–12 g/L of complex prebiotic oligosaccharides, substances that promote the growth of nonpathogenic commensals.
Several studies suggest that the supplementation of preterm formula with prebiotics may improve enteral tolerance. In a small, randomized, controlled trial of exclusively formula-fed preterm infants, the supplementation of formula with a mixture of 90% short-chain galacto-oligosaccharides (scGOS) and 10% long-chain fructo-oligosaccharides (lcFOS) reduced stool viscosity and resulted in faster gastrointestinal transit time than the same formula supplemented with placebo (2). A scGOS/lcFOS mixture has been shown to stimulate the growth of bifidobacteria and lactobacilli, reduce pathogens and result in a fecal pH, short-chain fatty acid pattern, and stool consistency similar to that of the breast-fed infant (3–5). In this study, we tested the hypothesis that a preterm formula containing 0.8 g scGOS/lcFOS mixture per dL (ratio, 9:1), when used to supplement insufficient maternal milk, improves enteral tolerance in preterm babies.
METHODS
Trial design and management.
We conducted a pragmatic United Kingdom, multicenter, prospective, double-blind, randomized, controlled study evaluating a prebiotic supplemented preterm milk formula within the context of current standard clinical practice including the promotion of breast feeding. The trial was approved by the UK Multicenter Research Ethics Committee, and the site specific research ethics review boards and Research & Development offices of 13 National Health Service hospitals (see Acknowledgments). Written informed parental consent was obtained. Trial conduct was overseen by a Steering Committee and independent Data Monitoring Committee (see Acknowledgments).
Participants.
Infants were eligible if born at a GA ≤32 wk 6 d and appropriately grown for GA [birth weight at or above the 10th centile for GA calculated using LMSGrowth (Harlow Healthcare: http://www.healthforallchildren.co.uk/)]. In addition, their mother agreed to the use of formula if she was unable or did not wish to breast feed or was unable to provide sufficient breast milk. Infants were ineligible if they had an immediate life-threatening congenital abnormality or any condition requiring major surgery.
Trial procedures.
Infants were randomized within 24 h of birth to receive maternal milk with either a standard preterm formula (Nutriprem 1; Cow and Gate) or an identical formula supplemented with scGOS/lcFOS to make up any shortfall in the volume required or as a sole feed if maternal milk was unavailable. A 24-h telephone randomization service was provided by the investigators (N.M. and S.U.) using a central computerized program written by the Statistical Advisory Service at Imperial College London. Randomization was performed in random blocks of 2–6, stratified by GA (≤28 wk 6 d and 29–32 wk 6 d) and recruiting hospital.
Trial formulas were supplied by Danone Research in 60 mL ready to feed containers marked only “A” or “B.” Formula was introduced only if maternal milk was unavailable or to make up a shortfall in the volume of milk required. Trial participation did not preclude the use of parenteral nutrition if considered clinically appropriate by the infant's physician. The control formula A, standard formula (SF), was an established standard preterm infant formula, Nutriprem 1. The experimental formula B (scGOS/lcFOS) was Nutriprem 1 supplemented with 0.8 g/100 mL scGOS/lcFOS in a 9:1 ratio. lcFOS is a fraction of inulin from chicory. Enzymatically synthesized scGOS has been described previously (5). The composition of the two formulae is shown in Table 1.
Enteral feed schedule.
Enteral feeds were commenced as early as possible within the first 24 h of birth. The rate of increase in milk volume was based on a predefined protocol taking into account the clinical status of the infant and the volume of gastric residuals. For infants born ≤28 weeks 6-d gestation, feeds were commenced at 0.5 mL/kg for every 2 h (rounded up to the nearest half milliliter) and were advanced daily by increasing 15 mL/kg to 15 mL/kg twice daily when a daily intake of 60 mL/kg was reached. For infants born 29 weeks to 32 weeks 6-d gestation, feeds were commenced at 15 mL/kg/24 h, increasing to 30 mL/kg/24 h on d 2 and by 15 mL/kg twice daily thereafter. Increments were based on birth weight until birth weight was regained, then on actual weight. Clinicians were at liberty to use parenteral nutrition and to increase or decrease the rate of advancement of milk feeds in kee** with the infant's clinical status. Suckling from the breast when the infant was sufficiently mature was encouraged and supported in accordance with standard practice. The number of days that there was any suckling at the breast was recorded. Infants were monitored until 40 wk postmenstrual age or discharge home, whichever occurred first.
Outcome measures.
As enteral tolerance in extremely preterm infants often fluctuates over time and length of stay is variable, the outcomes were designed to provide an assessment of immediate enteral tolerance and enteral tolerance throughout the neonatal period. The primary outcome (PO) was defined as the number of days from birth to establish a total daily enteral intake of 150 mL/kg. The principal secondary outcome (PSO) was defined as the proportion of days between birth and 28 d or discharge (whichever came first) that a total daily milk intake of at least 150 mL/kg was tolerated. Other secondary outcomes were as follows: 1) gain in weight, length, and head circumference (change in SD score between birth and 40 wk postmenstrual age or discharge); 2) fecal flora (colony-forming units/g stool of Bifidus species, Lactobacilli species, and pathogenic microorganisms) and fatty acid profile; 3) stool characteristics; 4) gastrointestinal signs; 5) fluid balance (the number of days between trial entry and 28 d where the serum sodium exceeded 148 mM or serum creatinine exceeded 150 mmol/L); 6) proven necrotizing enterocolitis according to predefined diagnostic criteria based on the study of Bell et al. (6), namely, abdominal distension and gastrointestinal bleeding, and on abdominal x-ray, either intestinal distension with bowel wall thickening or fixed bowel loops, pneumatosis intestinalis, portal vein gas, or confirmation at surgery or autopsy; 7) bloodstream infection according to predefined diagnostic criteria (7), namely, a blood culture positive for a recognized pathogen or a blood culture positive for coagulase-negative Staphylococcus, other skin commensal or mixed bacterial growth plus the acute onset of at least three of the following 10 clinical signs (increased oxygen requirement or ventilatory support; increase in apnea/bradycardia; hypotension; glucose intolerance; impaired peripheral perfusion (capillary refill time >3 s, pallor/mottling/core-peripheral temperature gap >2°C); lethargy/irritability/poor handling; temperature instability; ileus; fall in urine output (<1 mL/kg/h); and metabolic acidosis (base deficit >10 mM).
Data collection.
Case report forms (CRFs) were completed for all study participants by the local investigators and checked for completeness by the trial administrator. The Teleform questionnaire design, automated data entry and interpretation software package, was used to scan each CRF and transfer data into a spreadsheet. This software presents each variable from each CRF for on-screen operator checking before storage. All data management was undertaken at Imperial College London.
The volume of maternal milk and formula ingested, any prefeed gastric residual exceeding 4 mL/kg, posits or vomits after two consecutive feeds, marked or tender abdominal distension or presence of visible bowel loops, heavy bile staining of gastric aspirate or bilious vomit, whether the baby was “nil-by-mouth” postoperatively, level of care, and infant weight, if measured, were documented on a daily basis. Enteral intake per kilogram was calculated for each day (using weight for that particular day) and then averaged by the relevant number of days. Level-of-care categories were intensive, high dependency, and special care according to national UK definitions assigned by the British Association of Perinatal Medicine (http://www.bapm.org/publications/). All other evaluations were conducted on examination d 1–5 defined as follows: examination d 1, the day when the first enteral feed was administered; examination d 2, the first day of enteral feeding at a total volume of 150 mL/kg; examination d 3, d 28 of postnatal life; examination d 4, 37 wk postmenstrual age; examination d 5, 40 wk postmenstrual age or the day of discharge, whichever occurs earlier. Head circumference and length were documented on each of the 5 defined examination days.
Fecal evaluations.
The consistency of each stool sample on each of the 5 examination days was evaluated and recorded by the attending clinical staff on the basis of the appearance of the stool in the nappies. Stool characteristics were recorded as 1, watery; 2, soft; 3, seedy; 4, formed; 5, hard and frequency. The median of the scores obtained for each day was used to characterize daily stool consistency. On examination d 1 and d 3, two fecal samples were collected and stored at −20°C for later analysis for microbial composition and fatty acid profiling. Microbial composition was determined by FISH using specific 16S rRNA-targeted oligonucleotide probes (8). Amounts were expressed as percentage of the total number of bacteria.
Sample size estimation.
We previously observed a mean time of 12 d to attain an enteral intake of 150 mL/kg/d (SD of 4 d) in infants ≤32-wk gestation. A sample size of 73 or 86 infants in each group would have 85 and 90% power (5% significance level), respectively, to detect a difference of 2 d between the groups using the two-sided t test (PO one). As a potential benefit of scGOS/lcFOS is believed to be more rapid in intestinal transit time, we also noted a study by Mihatsch et al. (9) indicating that 50 infants per group were sufficient to demonstrate significantly faster establishment by 2 d of an intake 150 mL/kg/d in infants receiving a formula based on hydrolyzed protein when compared with formula based on intact protein. Taking these factors into consideration, we aimed to achieve a recruitment of 80 infants per group.
Data analysis.
Statistical analyses were performed at Imperial College London by the Trial Statistician (E.K.) using SPSS version 15. Analysis was by intention to treat. Summary measures are presented as median and interquartile range (IQR) and number (%) for continuous and categorical variables, respectively. The Mann-Whitney U test was used to compare continuous variables and the χ2 test for categorical variables between the two groups. Predefined subgroup analyses included analyses in GA subgroups and in subgroups with zero days nil-by-mouth postoperatively. A statistical modeling approach to data analysis [analysis of covariance (ANCOVA)] was prespecified. This uses the full dataset resulting in considerably more power than stratified subgroup analyses when numbers are small. ANCOVA was used for comparisons of the POs between the groups after adjustment for a predefined list of covariates, their interactions, and interaction with randomized group. This included proportion of time (to relevant endpoint) in level 1/intensive care, a complete course of antenatal steroids, proportion of days suckling at the breast, birth weight, GA at birth, z score for birth weight, type of first enteral feed, and average daily maternal milk intake. All covariates were included in the initial models for both POs. Nonsignificant covariates were excluded from the final ANCOVA models. To test the assumptions of ANCOVA, a series of diagnostic tests and plots were performed, including Shapiro-Wilks test for normality of the residuals. Log transformation was used if required.
RESULTS
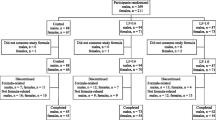
Details of recruitment and loss to analyses are shown in Fig. 1. One hundred sixty babies were recruited to this study. In six cases, parent consent was withdrawn postrandomization. In accordance with the requirements of the Research Ethics approval, no reason for withdrawal was given, and these babies were not included in the analyses. Five babies died (SF, 2; scGOS/lcFOS, 3), and of these, three did not receive any milk feeds and did not reach examination d 2, the point of PO assessment (SF, 1; scGOS/lcFOS, 2). An additional baby was withdrawn before reaching examination d 2, but in this case, the parents agreed to allow data previously collected to be included in the analysis. Therefore, there were 150 subjects available for PO analysis and 154 for PSO analysis. There were six adverse event reports filed, of which five were not considered related to the trial [renal failure, hyperkalemia, died before receiving any milk feeds (SF); high serum creatinine because of maternal cisplatin therapy (SF); withdrawal of intensive support after massive intracranial hemorrhage (scGOS/lcFOS formula); disseminated intravascular coagulation, pulmonary hemorrhage, died before receiving any milk feeds (scGOS/lcFOS formula); Gram-negative bloodstream infection, disseminated intravascular coagulation (SF), and one (SF) that was categorized by the local clinician as possibly related (abdominal distension and tenderness in association with Group B Streptococcal infection and possible milk curd obstruction)]. This infant made a full recovery. There were two protocol violations, in one instance where a baby was given nontrial formula in error over 3 d and in the other where a baby received the wrong trial formula for a single day.
Baseline characteristics.
There were no significant differences between the groups in baseline characteristics at trial entry or in the extent of parenteral nutrition use between the groups (Table 2). There were 21 babies <28-wk gestation in the SF group and 15 in the scGOS/lcFOS group. The median (IQR) length of stay was SF 34 d (22.5–49.5 d) and scGOS/lcFOS 31 d (21–43 d). Overall, 8% of infants in each group received maternal milk and 15% formula as sole enteral feed. There was no significant difference in the proportion of time that there was any suckling at the breast between the two groups [median (IQR): SF, 0.08 d (0–0.39 d); scGOS/lcFOS, 0.16 d (0.03–0.52 d); p = 0.12]. Eight babies in the SF group and two in the scGOS/lcFOS group had an episode of surgery with a significant difference between the groups in the number of days recorded as “postoperative/nil-by-mouth” (p = 0.01).
Primary outcome.
The median time to achieve a daily enteral intake of 150 mL/kg was longer in the SF than the scGOS/lcFOS group, but this was not statistically significant [median (IQR): SF, 7 d (6–9 d); scGOS/lcFOS, 6 d (5–8 d); p = 0.10]. As the distribution was skewed to the right, log transformation was used in further analyses. The ANCOVA model fitted to log-transformed time to achieve a daily enteral intake of 150 mL/kg showed that GA is the most important covariate for this outcome (p < 0.001), explaining 34.3% of total variation. The PO was 6% shorter in the group scGOS/lcFOS when compared with the group SF (Fig. 2), but this was not statistically significant (p = 0.34). The addition of average maternal milk intake explained only a further 5.2% of the total variation. This model was also fitted to the subset of babies with zero days nil-by-mouth postoperatively. The conclusions did not differ.
We also performed planned subgroup comparisons in the subgroup of babies <29-wk gestation (median weight, 1083 g; range, 615–1580; n = 36). All these babies except one (weighing 1580 g) was <1500 g. As with the analysis including all babies, there were no significant differences in the unadjusted PO [median (IQR): SF, 10 d (8–16 d); scGOS/lcFOS, 10 d (7.5–17.5 d); p = 0.94].
Principal secondary outcome.
There was no significant difference in the proportion of time from trial entry to 28 d/discharge during which a daily enteral intake of 150 mL/kg or greater was tolerated [median (IQR): SF, 0.48 d (0.23–0.68) d; scGOS/lcFOS, 0.45 d (0.25–0.63 d); p = 0.70). This was also the case in the subgroup of babies <29-wk gestation [median (IQR): SF, 0.35 d (0.04–0.66 d); scGOS/lcFOS, 0.41 d (0.036–0.59 d); p = 0.63]. The ANCOVA model for this outcome showed that SF versus scGOS/lcFOS group, GA, proportion of time in level 1 intensive care, maternal milk intake, and an interaction of randomized group with GA were all significant (Table 3). This model has an adjusted R2 of 28.1%. A higher proportion of time in level 1 intensive care was detrimental, and a higher maternal milk intake beneficial to enteral tolerance. Maternal milk intake explained 10% of the total variation. The main effect of the SF was lower enteral tolerance in comparison with scGOS/lcFOS. Taking this into consideration with the positive interaction with GA (Table 3; all babies; n = 154) indicates that enteral tolerance was lower in the SF group and was most marked the greater the degree of immaturity. The difference in enteral tolerance between the formula groups decreases with increasing maturity and disappears at a birth GA of ∼202 d (28 wk 6 d; Fig. 3). From the ANCOVA model, predicted enteral tolerance with the scGOS/lcFOS formula is 2.9% higher for a baby born at 196 d (28 wk) gestation and 9.9% higher at 26-wk gestation, but decreased or of no benefit in babies born >31 wk. We also evaluated only those babies in whom there were no days during which they were nil-by-mouth because they were postoperative. Infants were ineligible for recruitment if they had any condition requiring major surgery. Details of surgical procedures other than necrotizing enterocolitis were not collected, but the most frequent reasons for surgery in such a preterm population other than necrotizing enterocolitis are ligation of a patent ductus arteriosus, central vascular line insertion, and laser treatment for retinopathy of prematurity. The ANCOVA model was refitted to this subset (n = 144), but this did not change the conclusions (data not shown). In addition, the longer the time in the trial receiving formula, the more robust the evaluation of enteral tolerance, and for this, the cutoff chosen was 20 d. For this subset (n = 115), a beneficial effect is seen in babies up to a birth GA of 221 d (31 wk and 4 d). With the scGOS/lcFOS formula, predicted tolerance is 15.3% higher for a baby born at a GA of 196 d (28 wk) and 23.7% higher at 26 wk (Table 3, Fig. 3).
Predicted difference (95% confidence limits) by GA between randomized groups in PSO for all trial participants (solid line, dashed confidence limits) and for the subset with zero day postoperative/nil-by-mouth and 20 d or more from trial entry to discharge (long dash, dotted confidence limits). Model equations and mean squared errors are as follows: For all trial participants (n = 154), PSO = 1.933 − 1.009 × (Group = SF) − 0.542 × clevel1 − 0.007 × gestage + 0.001 × matmilk + 0.005× (Group = SF) ×gestation, MSE = 0.043. For the subset (n = 115), PSO = 2.240 − 1.329 × (Group = SF) − 0.634 × clevel1 − 0.008 × gestage + 0.001 × matmilk + 0.006 × (Group = SF) × gestation, MSE = 0.038. clevel1, proportion of time in level 1 intensive care; matmilk, average daily maternal milk intake.
Other secondary outcomes.
There were no statistically significant differences between the groups in gain in weight, length, or head circumference; fecal flora; gastrointestinal signs or indices of fluid balance; or in daily number of stools and stool characteristics on each of the 5 examination days. Although this difference was not statistically significant, the fatty acid profile (acetic, propionic, isobuteric, valeric, and lactic acid) showed >50% higher concentration of acetic acid on examination d 5 in the scGOS/lcFOS group when compared with the control group. In kee** with the significant negative correlation between the acetic acid concentration and stool pH (p = 0.014), the latter tended to be lower on examination d 5 in the scGOS/lcFOS group. Bifidobacterial counts were higher on examination d 3 and 5 in the scGOS/lcFOS group, but the differences were also not statistically significant.
There were three cases of necrotizing enterocolitis (SF, 1; scGOS/lcFOS, 2; p = 0.600), and 19 infants developed at least one episode of bloodstream infection (SF, 10; scGOS/lcFOS, 9; p = 0.180).
DISCUSSION
This was a pragmatic randomized trial aiming to reflect real clinical practice and promote the use of maternal breast milk. Formula was given only if maternal milk was insufficient, accounting for only one-third of the total enteral intake and with only 15% of babies receiving exclusive feeds of formula. As breast milk contains prebiotic oligosaccharides, this would have reduced any differences between the groups. No differences were found in the POs, but the study revealed a small but statistically significant and potentially clinically important improvement in enteral tolerance with increasing immaturity in the group randomized to the prebiotic formula, with no benefit or possibly even decreased benefit in babies >31-wk gestation. We accept that our study was not powered to detect differences in the PSO or differences in either outcome in the most immature infants, nor was it possible to anticipate the extent of provision of maternal milk and hence taken this into account when designing the study. These issues will have to be addressed in future trials.
Two principal mechanisms through which prebiotic substances are believed to influence enteral tolerance is through promotion of colonization with bifidogenic microbial species and more rapid gastrointestinal transit time (1,2). Nondigestible oligosaccharides serve as substrates for colonic fermentation by bifidobacteria and increase stool water content and decrease viscosity (2,10). Our study did not find statistically significant differences in fecal flora or stool characteristics, but this may also be attributable to the high proportion of maternal milk intake in both groups.
Sustained enteral tolerance can be problematic in preterm care. These infants are vulnerable to many and varied intercurrent illnesses, during which milk intake is compromised. Thus, initial advancement and maintenance of adequate intake throughout the period of hospitalization are important goals. The median time to reach a total daily enteral intake of 150 mL/kg was 1 d faster in the scGOS/lcFOS group though the trial was only powered to detect a difference of 2 d. Our sample size calculation was based on our previously observed mean time to reach an intake of 150 mL/kg/d of 12 d. Actual mean time to reach this intake in this study was 7 d for the SF group and 6 d for the scGOS/lcFOS group. To detect a difference of 1 d (80% power) would have required 253 babies in each arm. The overall improved tolerance over time is in kee** with historical trends generally observed in neonatal intensive care.
During the introduction and advancement of milk feeds, concurrent parenteral nutrition is often provided. Parenteral nutrition substantially increases the risk of bloodstream infection (11). In our study, 42% of babies receiving SF and 34% of babies receiving the scGOS/lcFOS formula received any parenteral nutrition. Although this was not a statistically significant difference, any stratagem that might reduce parenteral nutrition use by enhancing enteral tolerance is likely to have substantial ancillary benefit.
Intestinal integrity is vital to the defense against bacterial translocation and invasion that present clinically as bacteremia, blood stream infection, and necrotizing enterocolitis. The intestinal epithelium is dependent on commensal intestinal flora for maintenance of architectural integrity, response to insult, and ability to effect repair. Commensal bacteria release ligands, chemicals that interact with toll-like receptors (TLR) of the intestinal epithelium to induce sustained basal signaling that directs responses to injury and enhances repair (12). In experimental models, removal of TLR ligands disrupts signaling and leads to intestinal impaired resistance to injury (13). Human milk oligosaccharides seem to inhibit bacterial adhesion to epithelial surfaces and may have direct immunomodulatory properties by acting as ligands for selectins influencing inflammatory reactions (14). Conversely, the short-chain fatty acids that result from prebiotic fermentation might be deleterious to the immature intestinal epithelium (15). Short-chain fatty acids play a role in the regulation of intestinal motility (16) and this may account for more rapid gastric emptying observed in infants receiving prebiotic supplemented formula (17). Our study was not powered to detect differences in the rates of necrotizing enterocolitis and bloodstream infection and no significant differences were found. Exclusive feeding with human milk has been shown in high-quality meta-analyses to be protective against necrotizing enterocolitis although evidence in support of a protective role for partial human milk feeding is less certain (18). Human milk is unique in containing substantial quantities of complex oligosaccharides unlike milk of bovine origin (13). As preterm formulae derived from bovine milk have not hitherto been prebiotic supplemented, the lack of evidence of a protective role for partial breast feeding in previous studies may reflect the reduced ingestion of prebiotic oligosaccharides. Milk is often insufficient after preterm delivery, and the UK data from the Neonatal Data Analysis Unit (19) shows that only about one-third of infants admitted to neonatal units are discharged breast feeding, indicating that crucial issues for future investigation are whether prebiotic supplemented formula is confirmed to benefit enteral tolerance in the most immature babies, is protective against necrotizing enterocolitis and infection and the extent to which this translates into long-term benefit.
Abbreviations
- CRF:
-
case report form
- IQR:
-
interquartile range
- lcFOS:
-
long-chain fructo-oligosaccharides
- PO:
-
primary outcome
- PSO:
-
principal secondary outcome
- scGOS:
-
short-chain galacto-oligosaccharides
- SF:
-
standard formula
- TLR:
-
toll-like receptors
References
Indrio F, Riezzo G, Raimondi F, Bisceglia M, Cavallo L, Francavilla R 2008 The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr 152: 801–806
Mihatsch WA, Hoegel J, Pohlandt F 2006 Prebiotic oligosaccharides reduce stool viscosity and accelerate gastrointestinal transport in preterm infants. Acta Paediatr 95: 843–848
Boehm G, Fanaro S, Jelinek J, Stahl B, Marini A 2003 Prebiotic concept for infant nutrition. Acta Paediatr Suppl 91: 64–67
Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, Agosti M, Stahl B, Marini A, Mosca F 2005 Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr Suppl 94: 31–33
Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, Stahl B, Marini A 2002 Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed 86: F178–F181
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T 1978 Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187: 1–7
Modi N, Doré CJ, Saraswatula A, Bamford K, Richards MS, Coello R, Holmes A 2009 A case-definition for national and international neonatal bloodstream infection surveillance. Arch Dis Child Fetal Neonatal Ed 94: F8–F12
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW 2000 Analysis of intestinal flora development in breast fed and formula fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30: 61–67
Mihatsch WA, Franz AR, Högel J, Pohlandt F 2002 Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 110: 1199–1203
Cummings JH, Macfarlane GT 2002 Gastrointestinal effects of prebiotics. Br J Nutr 87: S145–S151
Holmes A, Doré CJ, Saraswatula A, Bamford K, Richards MS, Coello R, Modi N 2008 Risk factors and recommendations for rate stratification for surveillance of neonatal healthcare associated bloodstream infection. J Hosp Infect 68: 66–72
Madara J 2004 Building an intestine—architectural contributions of commensal bacteria. N Engl J Med 351: 1685–1686
Rakoff-Nahoum S, Pagliono J, Eslami-Varzaneh F, Edberg S, Medzhitov R 2004 Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241
Kunz C, Rudloff S, Baier W, Klein N, Strobel S 2000 Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 20: 699–722
Barrat E, Michel C, Poupeau G, David-Sochard A, Rival M, Pagniez A, Champ M, Darmaun D 2008 Supplementation with galacto-oligosaccharides and inulin increases bacterial translocation in artificially reared newborn rats. Pediatr Res 64: 34–39
Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ 2007 The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil 19: 66–74
Indrio F, Riezzo G, Raimondi F, Francavilla R, Montagna O, Valenzano ML, Cavallo L, Boehm G 2009 Prebiotics improve gastric motility and gastric electrical activity in preterm newborns. J Pediatr Gastroenterol Nutr 49: 258–261
Boyd CA, Quigley MA, Brocklehurst P 2007 Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 92: F169–F175
Neonatal Data Analysis Unit Report 2007–2009, Imperial College London 2010. Available at: http://www1.imperial.ac.uk/medicine/about/divisions/departmentofmedicine/infectiousdiseases/paediatrics/neonatalmedicine/ndau/reports/. Accessed July 13, 2010
Acknowledgements
We thank the contribution of the Preterm Prebiotic Study administrative team (Olivia Stevenson, Aparna Subaiah-Varma, and Louisa Lee); Sylvia Chalkley for data management; collaborating investigators; members of trial oversight committees; parent and infant participants; and the support from Guenther Boehm, Gilda Georgi, Jurgen Jellinek at Danone Research.
Trial Committees and additional investigators:
Steering: Dr. Nicholas Embleton (chair), Mrs. Caroline King, Dr. Pamela Cairns, Dr. Khalid Haque
Data Monitoring: Canon Ian Ainsworth Smith (chair), Dr. Mary Fewtrell, Professor RWI Cooke
Trial hospitals and investigators: Chelsea & Westminster (Dr. J. Fell, Dr. S. Uthaya, Professor N. Modi); Ealing (Dr. V. Chan); Epsom & St. Helier (Dr. K. Haque); Hillingdon (Dr. J. Menakaya); Mayday (Dr. J. Chang); Medway (Dr. A. Soe); Norfolk & Norwich (Dr. P. Clark); Northwick Park (Dr. R. Nicholl); Princess Royal (Dr. A. Long); Royal Hallamshire (Dr. R. Coombes); West Middlesex (Dr. D. Ratnasinghe); Whipps Cross (Dr. J. Ho); William Harvey (Dr. D. Long)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Danone Research, which provided an unrestricted research donation and lecture honorarium [to NM].
This work was supported by Danone Research which also provided an unrestricted research donation and lecture honorarium [to N.M.].
Rights and permissions
About this article
Cite this article
Modi, N., Uthaya, S., Fell, J. et al. A Randomized, Double-Blind, Controlled Trial of the Effect of Prebiotic Oligosaccharides on Enteral Tolerance in Preterm Infants (ISRCTN77444690). Pediatr Res 68, 440–445 (2010). https://doi.org/10.1203/PDR.0b013e3181f1cd59
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181f1cd59
- Springer Nature America, Inc.