Abstract
Background
A deficiency in alpha-1 antitrypsin (A1AD) leads to increased activity of proteolytic enzymes. The consequence is a damage of airways and alveoli and, ultimately, the development of emphysema and chronic obstructive pulmonary disease (COPD).
Purpose
Gender-specific differences in terms of comorbidities are still unclear due to the rarity of this genetic autosomal recessive disease.
Patients and methods
This retrospective observational study was conducted from January 1, 2005, to November 30, 2022, in the Department of Pneumology, HELIOS University-Clinic Wuppertal, University of Witten/Herdecke, Germany.
Results
Eleven patients with COPD due to A1AD could be included into the study (6 males, 54.5%; 95% CI 23.4–83.3%) with a mean age of 53.9 ± 11.6 years. The male study participants were of normal weight body mass index 24.17 ± 4.67, while the females were obese 31.2 ± 4.87 (p = 0.054). More women were smokers (60%, p = 0.567). Furthermore, all of the women had panlobular emphysema (100%, p = 0.455). All subjects suffered from COPD, with most male subjects in severe advanced stages (50%, p = 0.545). No case of liver involvement was observed in this study.
Conclusion
The findings of this study showed no statistically relevant gender-specific differences in comorbidities of patients with COPD due to A1AD.
Similar content being viewed by others
Background
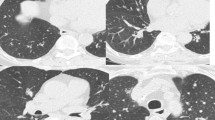
The rare hereditary disease alpha-1 antitrypsin deficiency (A1AD) leads to destruction of the air sacs in the lungs [1]. However, rapid diagnosis, symptomatic therapy, and replacement of the missing enzyme can significantly slow the progression of the disease. Due to a genetic defect, the enzyme A1AT is incorrectly formed in the liver [2]. The altered protein cannot be released from the liver into the bloodstream, which can damage liver tissue. This also causes a deficiency of A1AT in the lungs [2], where the enzyme protects the lungs from self-digestion through protein-splitting enzymes that are released by the body’s own defense cells to protect against bacteria. In the case of a large A1AD, the damage to the lungs includes attacks on the elastic tissue and destruction of particularly the alveoli [2]. The symptoms and consequences are similar to those of chronic obstructive pulmonary disease (COPD) caused by nicotine consumption, with which the disease is therefore often confused [3]. However, symptoms of A1AD typically appear at a younger age [4]. Moreover, in addition to pulmonary emphysema, bronchiectasis can also form [5].
Almost every person with COPD also has an accompanying disease affecting another organ system [6]. In the case of COPD, concomitant diseases of the heart and blood vessels, or risk factors to contract those diseases, are particularly common [7]. More than half of patients with COPD have vascular calcification or high blood pressure. Other common comorbidities include heart attacks, diabetes, and high cholesterol levels [8]. Additionally, over half of people with COPD have four or more comorbidities [6].
Different gender-specific conclusions have been postulated regarding the increased risk of men develo** COPD due to confounding with the risk factor of smoking [9]. Some studies have suggested an increased risk for men due to greater occupational and environmental exposures [10,11,12].
Therefore, the aim of the present study was to obtain more comprehensive and precise information about the comorbidities of patients with COPD due to A1AD in a sex comparison. For this purpose, all necessary data were collected on patients with COPD due to A1AD as determined by the International Statistical Classification of Disease (ICD E88.0) by conducting a search of the HELIOS Clinic Wuppertal database at the Witten/Herdecke University in Germany.
Material and methods
Study design and setting
We examined all data relevant to this retrospective observational study that were collected after treatment from the clinical database in the Department of Pneumology of the HELIOS Clinic Wuppertal for the period January 1, 2005, to December 31, 2022.
The HELIOS University Clinic Wuppertal is the largest hospital in the Bergisches Land in the state of North Rhine–Westphalia in Germany. It has 1051 beds and three locations, as well as 26 specialist departments. Since 2004, it has been the first university hospital run by a private operator in Germany. The clinic takes in around 50,000 inpatients and carries out around 100,000 outpatient treatments every year. The Department of Pneumology at the HELIOS Clinic in Wuppertal treats all types of lung diseases.
Alpha-1 antitrypsin deficiency
Alpha-1-antitrypsin deficiency is an inherited condition caused by a gene mutation in which the lungs and liver are damaged by low levels of the enzyme A1AT. An A1AD can lead to coughing, sputum production, and shortness of breath—initially with exertion, but later, also at rest. These signs are similar to the symptoms of other common lung diseases, such as asthma or COPD [13].
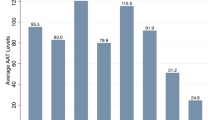
To measure the levels of A1AT in blood serum, a sample of blood is collected in a test tube, and to determine of the type of A1AT damage, the doctor draws blood from a fingertip or earlobe. Measuring A1AT levels in the blood is more time-consuming. After collection, the blood sample is sent to an external laboratory. If the A1AT concentration is low, an A1AD may be present [14].
Another option is to use a rapid test on site in the doctor’s office. This test enables A1AT to be ruled out quickly: After just 15 min, the doctor can find out, with a high degree of certainty, whether the patient has the greatest risk factor for A1AD, namely, the most common change in a particular allele—called the Z variant. It causes the protein A1AT to change and trigger an alpha-1 [15].
Chronic obstructive pulmonary disease (COPD)
COPD is a chronic, usually progressive, airway and lung disease characterized by airway obstruction that interferes with breathing and is not fully reversible after administration of bronchodilators. Diagnosis is by spirometry, and the forced expiratory volume in 1 s (FEV1) with COPD decreases annually as the disease progresses [16].
Pulmonary emphysema
Pulmonary emphysema is the chronic inflation of the lungs from air. This increases the volume of gas, making it difficult for the person affected by the disease to exhale. Typically, not all sections of the lung are involved [17].
Comorbidities
A concomitant disease is one that exists in a patient alongside an underlying disease that is the focus of therapy. It is also referred to as an additional diagnosis [18].
Statistical analysis
The mean values were calculated with the specification of the standard deviation for age, body mass index, pack years, length of hospital stay, and FEV1. These data were subjected to the independent-samples parametric T-test after checking for normality using the Kolmogorov–Smirnov test with Lilliefors correction. If the distribution of scores was not normal, the Mann–Whitney U-test was employed. Due to the small sample size of fewer than five observations, Fisher’s exact test was used to calculate the association between the two dichotomous categorical variables comparing comorbidities between sexes. A P-value < 0.05 was considered statistically significant.
Results
A total of 11 patients with COPD due to A1AD (6 male, 54.5%; 95%CI 23.4 − 83.3%) were found in the records of the Department of Pneumology for the HELIOS Clinic Wuppertal at the University of Witten/Herdecke in Germany in the study period of January 1, 2005, to December 31, 2022. The mean age of the study participants was 53.9 ± 11.6 years with no statistical differences between the two sexes (Table 1). There was no gender-related difference in the mean time for diagnosis, mean age at diagnosis, or mean age at symptom onset for A1AD (Table 1). A family screening for A1AD was not conducted in these patients in this study (Table 1). The male study participants were of normal weight, while the females were obese, but the difference was without statistical relevance. The number of female smokers was higher in this study, also without statistical significance (Table 1). Antitrypsin phenoty** was not recorded in all patients. However, without any relevance for this study, the proteinase inhibitor (Pi) PiZZ and PiMZ variants were common among the males (Table 1). In addition, all of the female participants with COPD exhibited the so-called panlobular emphysema (Table 1). All individuals in this study suffered from COPD, with most of the males in severe advanced stages of COPD, but without statistical significance (Table 1). Also without statistical significance, the male patients had more comorbidities, but there was no preponderance of one disease (Table 1). The female participants were prone to exacerbations of COPD. There was one death among the male study participants (Table 1).
Discussion
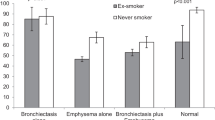
The results of this study showed no statistically relevant gender-specific differences in comorbidities in patients with COPD due to A1AD. A small number of the patients were found during the long follow-up period covered in this study. A1AD is a rare inherited disorder that has a major impact on quality of life and longevity in adults, especially in smokers [19]. A detailed analysis of this study found more smokers among the women, but one-third of the men were smokers. In smokers, A1AD leads to the development of emphysema. Because A1AT inhibits neutrophil elastase release, with a deficiency of A1AT, smoke-induced neutrophil elastase release is insufficiently inhibited. This leads to proteolytic damage to the pulmonary connective tissue, mainly from the elastic fibers, and the development of panlobular emphysema [20]. Therefore, patients with A1AD should stop smoking. All of the women in the present study had emphysema, compared to two-thirds of the men. Does gender really matter when it comes to this lung disease?
This disease was the first genetic risk factor described for COPD. More than 50 years since its description, new insights are still being provided into the more frequent occurrence of COPD in this rare disease. As already noted, the cause is a single genetic modification, and the clinical manifestations include emphysema, airway hyperreactivity, and bronchiectasis [20]. Indeed, the present study showed that all of the patients with A1AD developed COPD, with emphysema also often develo** and fewer incidences of bronchiectasis. According to the results of other studies, patients with A1AD often have an increased risk of develo** COPD and pulmonary emphysema [21, 22]. Deficiency of A1AT can progress to significant enzyme activity, which can lead to lung tissue disruption [21, 22]. The severity of COPD and emphysema can vary, typically manifesting in adulthood [21, 22]. Based on these findings, early diagnosis, regulated monitoring, and extensive treatment are definitely necessary in order to control the progression of this rare disease [21, 22].
The development of cirrhosis with A1AD is low [23], and no case of liver involvement was observed in this study, either. In addition, A1AD can cause liver disease according to former studies [24, 25]. The liver disease is ranging from mild liver enzyme abnormalities to more serious consequences such as liver cirrhosis [24, 25]. The liver complications can manifest in childhood or adulthood, which can lead to a different clinical presentation of A1AD [24, 25].
Develo** these comorbidities is increased in homozygous genotypes in individuals with A1AD. Patients in another study frequently experienced emphysema, bronchiectasis, and bronchial thickening, as well as early onset respiratory symptoms [26]. The most common genotype was PiZZ. Heterozygous genotypes and normal A1AD also showed significant lung disease [26]. Genetic counseling plays a critical role in understanding the diversity nature of A1AD [26]. Comprehensive treatment includes genetic testing for early detection, regular monitoring, and personalized treatment strategies [26]. Lifestyle changes, smoking cessation, and pulmonary rehabilitation are measures to improve the quality of life in this rare disease [26].
Many patients with advanced stages of COPD and emphysema have weight loss and a higher mortality rate. Many such patients were underweight in an earlier study [27]. However, underweight patients were not seen in this study. Instead, the male patients were of normal weight, and the women were overweight.
Some genetic variants of the proteinase inhibitor (Pi) A1AT have less serum concentration increases and are predisposed to liver disease and pulmonary emphysema. These deficiency variants can only be reliably diagnosed by phenoty**. While many genetic variants of A1AD are known, only a few are associated with a clinically relevant deficiency. The most common type of defect is the homozygous Z phenotype (PiZZ) [28].
The PiMZ phenotype of A1AT deficiency has emerged as key for in vivo studies of liver response, because synthesis and blocking of secretion occur simultaneously. In this study, as well, the PiMZ phenotype occurred frequently. However, phenoty** was not carried out in all patients, who had come to the hospital not only for clarification of this rare disease but also for other health reasons.
Patients with A1AD have been reported to have a higher prevalence of arterial hypertension, chronic kidney disease, and diabetes. They also have been shown to have more consultations and more frequent and longer hospital stays [29]. While we also observed the comorbidities in this study, a long hospital stay was not seen.
One study found that patients with A1AD have lower systolic and diastolic blood pressure, lower plasma triglycerides and residual cholesterol, a reduced risk of myocardial infarction, and a reduced risk of coronary artery disease [30]. These diseases were also under-represented in the present study.
Study limitations
Only a small number of patients with COPD due to A1AD could be found for analysis in this study because it was a single-center study. The information on comorbidities was based on the record in the files of the study site clinic. The small number of patients made the comparison of comorbidities based on sex more difficult. Genoty** data were only available for a minority of patients. Nonetheless, a correct segregation of individuals with severe A1AD probably occurred.
The normality tests compare the values in the sample to a normally distributed set of values with the same mean and standard deviation; the null hypothesis is that the sampling distribution is normal. For small sample sizes, there is little power to reject the null hypothesis, and thus, small samples are most likely to normality tests [31]. With large samples, even a small deviation from normality would produce significant results, although this small deviation does not affect the results of a parametric test.
Conclusions
This study showed no significant gender-specific differences in terms of comorbidities present with the very rare hereditary disorder A1AD.
Availability of data and materials
The authors confirm that all data generated or analyzed during this study are included in this article.
References
Udhaya Kumar S, Madhana Priya N, Thirumal Kumar D et al (2021) An integrative analysis to distinguish between emphysema (EML) and alpha-1 antitrypsin deficiency-related emphysema (ADL)—a systems biology approach. In: Donev R, Karabencheva-Christova T (eds) Advances in protein chemistry and structural biology, vol 127. Academic Press, Cambridge, MA, pp 315–342
Hazari YM, Bashir A, Habib M et al (2017) Alpha-1-antitrypsin deficiency: genetic variations, clinical manifestations and therapeutic interventions. Mutat Res Rev Mutat Res 773:14–25
McElvaney OJ, Cleary B, Fraughen DD et al (2022) Attitudes towards vaccination for coronavirus disease 2019 in patients with severe alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis 9(2):266–273
McElvaney NG, Stoller JK, Buist AS et al (1997) The α1-Antitrypsin Deficiency Registry Study Group. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of α1-antitrypsin deficiency. Chest. 111(2):394–403
Huang YT, Wencker M, Driehuys B (2021) Imaging in alpha-1 antitrypsin deficiency: a window into the disease. Ther Adv Chronic Dis. 12_suppl:20406223211024524
Kim-Dorner SJ, Schmidt T, Kuhlmann A, Graf von der Schulenburg JM, Welte T, Lingner H (2022) Age- and gender-based comorbidity categories in general practitioner and pulmonology patients with COPD. NPJ Prim Care Respir Med 32(1):17
Yin HL, Yin SQ, Lin QY, Xu Y, Xu HW, Liu T (2017) Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine 96(19):e6836
Cavaillès A, Brinchault-Rabin G, Dixmier A et al (2013) Comorbidities of COPD. Eur Respir Rev 22(130):454–475
Aryal S, Diaz-Guzman E, Mannino DM (2013) COPD and gender differences: an update. Transl Res 162(4):208–218
Grahn K, Gustavsson P, Andersson T et al (2021) Occupational exposure to particles and increased risk of develo** chronic obstructive pulmonary disease (COPD): a population-based cohort study in Stockholm. Sweden Environ Res 200:111739
Hart JE, Laden F, Eisen EA, Smith TJ, Garshick E (2009) Chronic obstructive pulmonary disease mortality in railroad workers. Occup Environ Med 66(4):221–226
Bergdahl IA, Torén K, Eriksson K, Hedlund U, Nilsson T, Flodin R, Järvholm (2004) Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J. 23(3):402–406
Kelly E, Greene CM, Carroll TP, McElvaney NG, O’Neill SJ (2010) Alpha-1 antitrypsin deficiency. Respir Med 104(6):763–772
Gökhan P, Sema A (2018) Evaluation of alpha-1-antitrypsin levels in blood serum of patients with chronic obstructive pulmonary disease. Acta Biomed 90(1):37–43
Jardim JR, Casas-Maldonado F, Fernandes FLA, Castellano MVCO, Torres-Durán M, Miravitlles M (2021) Update on and future perspectives for the diagnosis of alpha-1 antitrypsin deficiency in Brazil. J Bras Pneumol 47(3):e20200380
Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L (2007) Definition, epidemiology and natural history of COPD. Eur Respir J 30(5):993–1013
Thurlbeck WM, Müller NL (1994) Emphysema: definition, imaging, and quantification. Am J Roentgenol 163(5):1017–1025
Moffat K, Mercer SW (2015) Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract 16:129
de Vos JD, Hillberg O, Perch M, Jensen JU, Wilcke JT, Løkke A (2021) Alpha-1-antitrypsin deficiency. Ugeskr Laeger. 183(30):V02210150. Danish
Abboud RT, Ford GT, Chapman KR (2005) Emphysema in α1-antitrypsin deficiency: does replacement therapy affect outcome? Treat Respir Med 4(1):1–8
Sandhaus R, Strange C, Stone G et al (2020) Comorbidity associations with AATD among commercially insured and Medicare beneficiaries with COPD in the US. Int J Chron Obstruct Pulmon Dis 15:2389–2397. https://doi.org/10.2147/COPD.S263297. PMID:33116454; PMCID: PMC7547287
Wells AD, Woods A, Hilleman DE, Malesker MA (2019) Alpha-1 antitrypsin replacement in patients with COPD. P T. 44(7):412–415. PMID: 31258312; PMCID: PMC6590928
Cosme A, Ojeda E, Torrado J, Carrera A, Castiella A, Zapata E (2003) Alteraciones hepáticas por déficit de alfa-1-antitripsina en adultos. Estudio de 5 pacientes y análisis de los casos publicados en la bibliografía española [Liver alterations due to alpha-1-antitrypsin deficiency in adults. Study of 5 patients and analysis of the cases reported in the Spanish literature]. Gastroenterol Hepatol. 26(4):251-256. Spanish
Mitchell EL, Khan Z (2017) Liver disease in alpha-1 antitrypsin deficiency: current approaches and future directions. Curr Pathobiol Rep. 5(3):243–252. https://doi.org/10.1007/s40139-017-0147-5. Epub 2017 Jul 10. Erratum in: Curr Pathobiol Rep. 2018;6(1):97. PMID: 29399420; PMCID: PMC5780543
Patel D, McAllister SL, Teckman JH (2021) Alpha-1 antitrypsin deficiency liver disease. Transl Gastroenterol Hepatol 6:23. https://doi.org/10.21037/tgh.2020.02.23. PMID: 33824927; PMCID: PMC7829072
Felisbino MB, Fernandes FLA, Nucci MCNM et al (2018) The patient profile of individuals with alpha-1 antitrypsine gene mutations at a referral center in Brazil. J Bras Pneumol 44(5):383–389. https://doi.org/10.1590/S1806-37562017000000420. PMID:30517339; PMCID: PMC6467596
Seersholm N (1997) Body mass index and mortality in patients with severe α1-antitrypsin deficiency. Respir Med 91(2):77–82
de Serres FJ (2002) Worldwide racial and ethnic distribution of α1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest 122(5):1818–1829
Greulich T, Nell C, Hohmann D et al (2017) The prevalence of diagnosed α1-antitrypsin deficiency and its comorbidities: results from a large population-based database. Eur Respir J 49(1):1600154
Winther SV, Ahmed D, Al-Shuweli S et al (2022) Severe α1-antitrypsin deficiency associated with lower blood pressure and reduced risk of ischemic heart disease: a cohort study of 91,540 individuals and a meta-analysis. Respir Res 23(1):55
Le Boedec K (2016) Sensitivity and specificity of normality tests and consequences on reference interval accuracy at small sample size: a computer-simulation study. Vet Clin Pathol 45(4):648–656
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
YJ made significant contributions to the reported work, be it in conception, study design, conduct, data collection, analysis, writing, and interpretation. RK revised and critically reviewed the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Witten/Herdecke University in Witten, Germany, approved this study. The legal basis for processing of the relevant personal data was obtained from each study participant after they provided voluntary written consent, in accordance with the European General Data Protection Regulation. All personal data from all study participants was removed prior to data processing.
Consent for publication
We confirm that all materials included in this manuscript can be published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yayan, J., Rasche, K. No gender-specific differences in comorbidities in patients with chronic obstructive pulmonary disease due to alpha-1 antitrypsin deficiency. Egypt J Bronchol 17, 73 (2023). https://doi.org/10.1186/s43168-023-00251-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00251-0