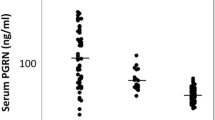Abstract
Background
Pulmonary involvement is still regarded as a common cause of morbidity in Polymyositis/Dermatomyositis. Interstitial lung disease can result in potentially fatal consequences such as ventilatory failure, secondary pulmonary arterial hypertension, or cor pulmonale. Early diagnosis of interstitial lung disease is hence a top priority in Polymyositis/Dermatomyositis patients. Krebs von den Lungen-6 is a transmembrane mucoprotein that has recently been identified as a promising marker for interstitial lung disease diagnosis and progression. As a result, it is regarded as a powerful predictor of interstitial lung disease severity. Thirty polymyositis/dermatomyocitis patients were enrolled in this study. Thirty age and sex matched healthy individuals were selected as control group. Cutaneous Dermatomyositis Disease Area Severity index(CDASI) was used for evaluation of skin severity, KL6 was measured using Elisa kit, High-resolution computed tomography, pulmonary function tests were made.
Results
Sixteen female and 14 male patients had a mean age of 41.64–8.02 and amedian of the disease duration of 4 years. Fifteen patients (50%) had normal readings on High-resolution computed tomography of the chest HRCT chest, while the other half had significant HRCT chest findings. KL-6 concentrations were seen to be higher in Polymyositis/Dermatomyositis patients mainly those with ILD (mean ± SD 38.66 ± 22.98), compared to the control group (Mean ± S 589.04 ± 409) and in patients without ILD (Mean ± SD 86.70 ± 8.99), (p < 0.001). KL-6 serum concentrations were shown to have a significant connection with the HRCT score (r = 0.803, P < 0.001). Forced vital capacity (FVC%) (r = − 0.910, P < 0.001), forced expiratory volume in 1 s (FEV1%) (r = − 0.767, P < 0.001), and FEV1/FVC% (r = − 0.228, P = 0.112) were all inversely related to KL-6 concentrations.
Conclusion
Individuals with PM and DM with concomitant ILD have increased KL-6 serum concentrations in comparison to myositis individuals without ILD, denoting its potential role in diagnosis and follow up of PM/DM with ILD. Further studies are needed to discuss the role of KL6 in large sample of the patient's population, and its correlation with other organ affection rather than ILD. Also to clarify the potentiality of adding the KL6 biomarker to the guidelines of treat to target for Rheumatic diseases with lung affection and to see the effect of treatment on KL6 serum level.
Similar content being viewed by others
Background
Dermatomyositis is an autoimmune disease of muscle and skin with genetic predisposition; it is 5 to 22 per 100,000 people prevalence with a male-to-female ratio of 1:3 and frequency ranges between 1.2 and 19 per million at-risk individuals per year [1]. Proximal muscle weakness is the main domain in inflammatory myositis while major organ affection is a concern. Interstitial lung inflammation with subsequent shortness of breath is a major comorbidity. So, early detection and management are necessary for good outcomes and prognosis [2].
Increasingly, ILD’s severity and progression during its clinical course could happen at any stage that is strongly linked to considerable morbidity and mortality [3]. ILD prevalence ranges from 9-78% worldwide [4]. ILD prevalence ranges from 10.7 to 27.14 per 100,000 individuals worldwide [4].
The earliest clinical sign of a systemic autoimmune illness may be lung [5].
General interstitial pneumonia, organizing pneumonia, nonspecific interstitial pneumonia, lymphocytic interstitial pneumonia, and diffuse alveolar harm are the most prevalent patterns of ILD histology in lung biopsies associated with myositis [6]. By concentrating on fibrotic alterations, HRCT scans provide the opportunity to evaluate illness severity with greater precision and sensitivity [7].
KL-6 is a glycoprotein with high molecular weight mostly associated with type II alveolar epithelial cells (AECs) and the MUC1 gene encodes it [8].
ILD’s clinical course is incredibly unexpected. As, the inflammatory storm may rupture a disulfide link close to type II AEC membranes, allowing KL-6 diffusion into pulmonary epithelial lining fluid and blood flow, as a result, increase KL-6’s predictive value to identify those who are more prone to experience advanced fibrosis as well as some unfavorable ILD outcomes [8].
The purpose of this work: Was to check for the KL-6 marker existence in the sera of individuals with polymyositis and dermatomyositis (PM and DM) and determine whether there is a significant relationship with clinical, laboratory, and radiological parameters and with ILD severity.
Patients and methods
This cross-sectional study is performed on 30 patients that met dermatomyositis Bohen and Peter’s 1975 dermatomyositis classification criteria [9]. The Research cohort was drawn from inpatient and outpatient Rheumatology and Rehabilitation, Dermatology, and Pulmonology clinics at university hospitals of different governorates (Benha university hospitals and Kafer -Elsheikh university hospitals). Thirty healthy individuals; age and sex-matched patients represented a control group. Patients were divided into groups: group1 fifteen patients suffered from ILD, group11 Fifteen patients without ILD Inclusion crieteria: Thirty DM and PM cases were above the age of 16, their disease duration was more than 1year. Patients who were complaining proximal muscle weakness, and those who were suffering from interstitial lung affection, either symptomatic or asymptomatic, all were included. All patients were on treatment protocol, including immunosuppressive drugs, steroids. Exclusion crieteria: Those under the age of 16 with proximal muscle weakness, cases of distal muscle weakness and/or muscle weakness due to neurological causes, infections, chronic diseases complicated by proximal weakness (endocrine disorders, chronic liver disease, and renal disease), other chronic lung pathologies, a history of consuming drugs that cause proximal muscle weakness (HMGCOA inhibitors like statin, steroid intake for other conditions, colchicine intake), pregnant women all were eliminated before starting the study. Fifty healthy subjects; age and sex-matched patients represented a control group.
The local ethical committee of the faculty of medicine granted approval for the study. All participants provided written consent after being fully informed MS-7–2020.
For all participants, clinical, laboratory, and radiographic evaluations by expert radiologist blinded to the clinical data were performed, all study participants underwent a history-taking process, the general examination that looks at the body’s systems, vital signs, musculoskeletal evaluation, and cardiopulmonary examination looking for signs of hypoxia, pulmonary hypertension with subsequent comorbidities like congested RT side heart failure.
Lab investigation
Creatinine kinase(CK U/L ) is generally considered the most sensitive indication of inflammatory myopathy. (LDH U/L, AST U/L, and ALTU/L) but are less sensitive. Measurements of acute phase reactants (ESR mm/h, CRP mg/L), and immunological profile (anti-Jo-1 (antisynthetase)) finding is common in those with ILD. Measurement of KL-6 serum level (normal range is less than 500 U/ml), KL-6 was measured utilizing ELISA kits following the manufacturer’s instructions.
Test principle
The kit employs an enzyme-linked immunosorbent assay (ELISA) based on the double-antibody sandwich method to measure the concentration of Human Krebs von den Lundgen-6 (KL-6) in samples. Before analysis, serum from patients was kept at − 80°. Add Krebs von den Lundgen-6 (KL-6) to monoclonal antibody Enzyme well that is pre-coated with Human Krebs von den Lundgen-6 (KL-6) monoclonal antibody, incubation; then, add Krebs von den Lundgen-6 (KL-6) antibodies labeled with biotin, and form an immune complex by combining Streptavidin-HRP; then repeat the incubation and washing steps to eliminate the unreacted enzyme. After adding Chromogen Solution, A and B, the liquid undergoes a color change from blue to yellow due to the acidic conditions. There is a positive correlation between the intensity of the color and the concentration of the Human Substance Krebs von den Lundgen-6 (KL-6) in the sample.
Radiology
CT scanning is useful in the evaluation of potential malignancies that might be associated with inflammatory myopathy. HRCT chest score is……………….. In which Six anatomically diverse thoracic HRCT axial segments were chosen for the investigation [10]. The honeycombing (H), reticulation (R), traction bronchiectasis (TB), and ground glass opacification with TB (GGO + TB, indicating fine fibrosis) presence in both the left and right sections was assessed and assigned a score rounded to the nearest 5%. Total fibrosis score (TFS) was calculated as the sum of scores for every fibrotic component (R, H, ground glass with TB and TB). All abnormalities observed in the CT scan were evaluated according to the standardized terms established by the Fleischner Society (Fig. 1) [11].
HRCT chest window of 49 years old dermatomyositis female complaining dry cough, shortness of breathing 1 year ago, lab investigations were (ESR: 74 mm/h, CRP: 26 Mg/L, LDH: 842 U/L, AST: 131 U/L, CPK: 4581 U/L, KL-6: 921 U/ML, positive ANA, AJOI: showed bilateral GGO, fibrosis, and bronchiectasis on both lower lung zones
Lung function tests
Pulmonary function tests are crucial for providing a respiratory involvement objective evaluation, (FVC (L), FEV1%, FEV1/ FVC% ratio) [12].
Muscle biopsy
Muscle biopsies (deltoid or quadriceps femoris) are crucial for aiding in the diagnosis of polymyositis. MRI and EMG may be used to determine the biopsy spot. By utilizing the contralateral side, avoid biopsies of locations previously investigated with EMG.
Statistical analysis
The data was inputted and analyzed using IBM SPSS software version 20.0. Descriptive statistics included number, percentage, range, mean, standard deviation, median, and interquartile range (IQR). The normality of distribution was assessed using the Shapiro–Wilk and Kolmogorov–Smirnov tests. A 5% significance threshold was used. The chi-square test compared categorical variables, while the t-test compared normally distributed quantitative variables between two groups. The Mann–Whitney test compared quantitative variables with anomalous distribution, and the Kruskal–Wallis test compared more than two groups. Spearman coefficient determined the correlation between abnormally dispersed quantitative variables. Receiver operating characteristic (ROC) curves graphed sensitivity and specificity at various cut-off values. The area under the ROC curve represented diagnostic performance. Sensitivity detected true positives, while specificity eliminated true negatives. Positive predictive value (PPV) indicated disease probability in individuals with positive test results, and negative predictive value (NPV) indicated disease absence in individuals with negative test results.
Results
Thirty patients satisfied the inclusion criteria of PM and DM, their mean age (41.6 ± 48.02), 16 females 52% and 14 males 48% (F: M 1.1:1), 17 females 52% and 13 males 48% in the control group (F: M 1.2:1), with mean age (40.82 ± 6.64). The median (IQR) disease duration was 4 years. Both laboratory and clinical features are mentioned in Table 1.
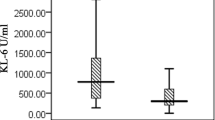
KL-6 serum concentrations were elevated in the myositis group than in healthy controls (P < 0.001) as seen in Table 2. Active PM/DM individuals with ILD had greater levels of KL-6 (mean SD 1080.0 ± 138.08, P < 0.001) than those without ILD (mean ± SD 86.70 ± 8.99, P < 0.001), The mean serum KL-6 level is (589.04 ± 409). The mean HRCT score is (12.08 ± 7.12) (Table 3). Strong positive associations were seen between KL-6 and laboratory variables (CPK, ESR, CRP, anti-Jo-1) (r = 0.897, P < 0.001), (r = 0.846, P < 0.001), (r = 0.918, P < 0.001), (u = 115.0 p < 0.001), respectively, while negative correlation was found with LDH (rs − 0.228, p = 0.111) (Table 4). HRCT chest scores showed highly statistically significant correlations with KL-6 in DM/PM subjects (Table 5). Lung functions and KL-6 have a strong, substantial association. Statistically significant negative correlations were detected between KL-6 and FVC′ (L), FVC%, FEV1 (L), FEV1% (P < 0.001, r = − 0.845), (P < 0.001, r = − 0.910), (P < 0.001, r = − 0.778), (P < 0.001, r = − 0.767) (Figs. 2, 3, 4, 5 and 6). The ROC curve study produced a good AUC of 0.991, a KL-6 cut-off value of more than 76 U/ml, sensitivity of 96–98%, specificity of 80–90%, positive predictive value of 83.1%, and negative predictive value of 95.7% (Fig. 6).
Discussion
ILD is distinguished by diffuse pulmonary infiltrates, restricted lung function impairment, and reduced carbon monoxide diffusing capacity (DLco), is one of morbidity and mortality main causes in myositis individuals. According to Fathi et al., a prompt and precise ILD diagnosis is essential to stop the progression of the condition from the initial inflammatory activity (alveolitis) to the end-stage disease with irreversible honeycombing and fibrosis [13].
Serum KL-6 concentrations were substantially lower in healthy controls compared to the myositis group (38.66 ± 22.98 vs 589.04 ± 409.55 U/ml; P < 0.001).
This was consistent with Kubo et al. study which provided data about the serum KL-6 concentrations in PM/DM individuals they were substantially greater than those in healthy controls (1183 ± 310 vs. 383 9 U/ml; P < 0.01) [14].
Additionally, Fathi et al. discovered that PM or DM individuals had elevated KL-6 serum concentrations than healthy controls: 400 (range: 132–2318) versus 225 (range: 136–519) U mL1 (P = 0.01) [12].
In this research, serum KL-6 concentration fluctuates between patients as regard CDASI, whereas greater levels seem to be associated with severe skin affection more than cases with a mild skin condition (mean 1080.0 ± 138.08, p < 0.001*) (mean 148.50 ± 22.61, p < 0.001), respectively.
Additionally, there is a highly statistically significant inverse relationship between muscle power grading and KL-6 serum concentrations. Individuals with lower muscle power grade (grade 0) have greater mean levels of KL-6 (1175.0 ± 176.8, p < 0.001*) than patients with higher grades (muscle power grade 4) (median 103.9 ± 37.25, p < 0.001*), in contrast to Bandoh et al. who were unable to link KL-6 serum concentrations to muscle enzymes [15].
Also, patients who exhibit high signal intensity on STIR, and magnetic resonance imaging (MRI) activities have serum levels of KL-6 that are higher than those who have chronic muscle injury and atrophy. In addition, individuals with active inflammation in their biopsy samples had greater blood KL-6 levels than those who had chronic myositis with atrophy and fibrosis (mean ± SD, 117.60 ± 35.86), (Mean ± SD, 903.33 ± 164.61) consecutively. Patients with positive immunological profiles (ANA and anti-Jo-1) have greater levels of KL-6 than individuals with negative anti-Jo-1. Also, in this investigation, a favorable association between blood KL-6 and CPK concentrations in myositis individuals has been established.
Contrary to Targoff et al.’s findings, which revealed no association between increased blood KL-6 concentrations and other laboratory or clinical characteristics involving muscle weakness, serum CK activity, or skin eruptions [16].
Also, according to data from this study focusing on lung parameters, active PM/DM individuals with ILD had greater KL-6 concentrations than those with no ILD (mean ± SD 1080.0 ± 138.08, P 0.001) (mean ± SD 86.70 ± 8.99, P < 0.001).
As documented by Oguz et al., the median KL-6 concentrations in 113 CTD individuals and 45 healthy controls were greater in the ILD group than in those without or healthy subjects (p < 0.008) [17].
Additionally, the same was revealed by Chaojun et al. who observed that idiopathic inflammatory myositis individuals with ILD had substantially greater KL-6 serum concentrations in comparison to those without ILD (776.5 [372.3–1378.8] vs. 297.5 [204.75–599.3] U/ml, P < 0.001) when they evaluated KL-6 serum concentrations in 184 individuals utilizing a chemiluminescent enzyme immunoassay [18].
KL-6 has recently been hailed as a promising biomarker for ILD [19]. Therefore, an elevation in KL-6 concentrations may indicate a rise in the number of regenerating type 2 pneumocytes as a result of pulmonary damage [20].
Lung’s pulmonary function tests and HRCT are common diagnostic tools used to check for ILD in myositis patients. To determine if ILD is severe enough to warrant early therapeutic intervention, pulmonary function tests, and radiographic characteristics are employed.
It was observed that KL-6 serum concentrations were greater in patients with high-grade lung fibrosis on HRCT compared with moderate affection (ground glass opacities and traction bronchiectasis) and mild affection (ground glass opacities), KL-6 concentrations were considerably greater in patients with ILD than those without ILD U/ml, and with activity (p < 0.001).
Lee et al. revealed that KL-6 concentrations had strongly correlated with HRCT findings, some clinical variables in connective tissue disease (CTD)-ILDs, so used as a potential tool for assessment of CTD-ILD severity [21].
KL-6 expression and inflammatory cascade activation in type II AECs may cause lung fibrotic alterations that indicate more severe lung harm and progressive abnormalities in lung imaging. According to a research by Xu L et al., damaged AECs start fibroblast to myofibroblast differentiation, boost the expression of type I and type III collagen, and stop the generation of HGF via KL-6 release. Therefore, the fibrogenesis and epithelial-mesenchymal transition in ILD are mediated by KL-6, and fibrotic HP patients have greater KL-6 levels [22].
There are two basic causes for the increase in KL-6 levels, that may effectively distinguish the severity and different phases of HP. pulmonary vascular epithelial damage and diffuse alveolar epithelial cell injury are the most prevalent kinds. The basic pathogenic mechanism of alveolar epithelial cell damage is chronic fibrosis development [23]. When lung mesenchymal or parenchymal epithelial cells are harmed, the basement membrane integrity of lung capillaries is compromised, resulting in an elevation in lung capillary permeability. To heal the injured type I alveolar epithelial cells, type II alveolar epithelial cells multiply considerably, resulting in an elevation of produced KL-6 entering the blood via the lungs. KL-6 might enhance the buildup of extracellular matrix in HP patients' lungs, leading to fibrosis [24]. Elhai discovered high KL-6 serum levels when evaluating IPF severity [20].
KL-6 has been linked to ILD pathophysiology by increasing pulmonary fibroblast proliferation and migration; it is highly excreted in the serum, BAL fluid, and ILD patients’ lung tissue. These findings suggest that higher KL-6 concentrations in peripheral blood represent the pathological features of widespread alveolar injury in the interstitial milieu [7].
In the current study, KL-6 blood concentrations were inversely related to PFTs (FVC (rs = − 0.845, p < 0.001*), FEV1 (rs = − 0.778, p < 0.001), and FEV1/FVC% (rs = − 0.228, p = 0.112).
In line with these results, Lee et al. who studied 165 CTD-ILD individuals (41 rheumatoid arthritis, 53 cases of systemic sclerosis, 56 inflammatory myopathies, 15 systemic lupus erythematosus or SJS) reported that KL-6 serum levels had a negative correlation with both DLCO% (r = − 0.578, p < 0.001) and FVC% (r = − 0.399, P < 0.001) [21].
Furthermore, Bonella et al. KL-6 levels were negatively affected by FVC% and DLCO% changes [25]. The study by Sokai et al. revealed that KL-6 concentrations are correlated with a considerable drop in FVC and DLCO percent [26].
According to Kumanovics et al. study which was carried out on 135 patients with connective tissue disease-related interstitial lung pathology (31 with myositis,104 with systemic sclerosis). KL-6 levels were inversely connected with FVC (r = − 0.32, p 0.001) and DLco (r = − 0.55, p 0.001) [27].
In agreement with the findings of Silvia Sánchez-Díez et al., serum KL-6 concentrations were negatively correlated with total lung capacity and carbon monoxide diffusing capacity in a study carried out on 34 patients with fibrotic h hypersensitivity pneumonitis in comparison to 15 patients with non-fibrotic HP [28].
Serial KL-6 concentration changes have been connected to ILD development [29]. For an accurate diagnosis, complete clinical evaluation, pulmonary function testing, and HRCT are required, in addition to the observation of high KL-6 values [30].
Nevertheless, considering the small sample size of the individual investigations, more studies are required for results verification.
Limitations
The relatively low number for screening ILD in PM/DM that may interfere with the statistical analysis accuracy and build up reliable results represent a limitation in this study.
Conclusion
Individuals with PM and DM with concomitant ILD have increased KL-6 serum concentrations in comparison to myositis individuals without ILD, denoting its potential role in diagnosis and follow-up of PM/DM with ILD. Further studies are needed to discuss the role of KL6 in large sample of the patient's population, and its correlation with other organ affection rather than ILD. Also to clarify the potentiality of adding the KL-6 biomarker to the guidelines of treatment to target for rheumatic diseases with lung affection and to see the effect of treatment on KL-6 serum level.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Anti-Jo-1:
-
Anti synthetase antibody
- ALT:
-
Alanine Aminotransferase
- ANA:
-
Antinuclear antibody
- AST:
-
Aspartate aminotransferase
- CDASI:
-
Cutaneous disease area and severity index
- CPK:
-
Creatinine phosphokinase
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- GGO:
-
Ground glass opacity
- HRCT:
-
High-resolution CT scan
- ILD:
-
Interstitial lung disease
- KL-6:
-
Von den Lugen 6
- LDH:
-
Lactate dehydrogenase
- TB:
-
Traction bronchiectasis
References
Cheeti A, Brent LH, Panginikkod S. Autoimmune Myopathies. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
Hervier B, Uzunhan Y (2019) Inflammatory myopathy-related interstitial lung disease: from pathophysiology to treatment. Front Med (Lausanne) 6:326
Usui Y, Kaga A, Sakai F, Shiono A, Komiyama K, Hagiwara K, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open. 2013;3(7).
Shappley C, Paik JJ, Saketkoo LA (2019) Myositis-Related Interstitial Lung Diseases: Diagnostic Features, Treatment, and Complications. Curr Treatm Opt Rheumatol 5:56–83
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD (2011) Pulmonary manifestations of systemic autoimmune diseases. Maedica (Bucur) 6(3):224–229
Mathai SC, Danoff SK (2016) Management of interstitial lung disease associated with connective tissue disease. BMJ 352:h6819
Fujisawa T, Hozumi H, Kono M, Enomoto N, Nakamura Y, Inui N et al (2017) Predictive factors for long-term outcome in polymyositis/dermatomyositis-associated interstitial lung diseases. Respir Investig 55(2):130–137
Kondo T, Hattori N, Ishikawa N, Murai H, Haruta Y, Hirohashi N et al (2011) KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res 12(1):32
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7):344–347
Edey AJ, Devaraj AA, Barker RP, Nicholson AG, Wells AU, Hansell DM (2011) Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur Radiol 21(8):1586–1593
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697–722
Gold WM, Koth LL. Pulmonary function testing. Murray and Nadel's Textbook of Respiratory Medicine. 2016:407.
Fathi M, Barbasso Helmers S, Lundberg IE (2012) KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 271(6):589–597
Kubo M, Ihn H, Yamane K, Kikuchi K, Yazawa N, Soma Y et al (2000) Serum KL-6 in adult patients with polymyositis and dermatomyositis. Rheumatology (Oxford) 39(6):632–636
Bandoh S, Fujita J, Ohtsuki Y, Ueda Y, Hojo S, Tokuda M et al (2000) Sequential changes of KL-6 in sera of patients with interstitial pneumonia associated with polymyositis/dermatomyositis. Ann Rheum Dis 59(4):257–262
Targoff IN (1993) Humoral immunity in polymyositis/dermatomyositis. J Investig Dermatol 100(1):S116–S123
Oguz EO, Kucuksahin O, Turgay M, Yildizgoren MT, Ates A, Demir N et al (2016) Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 35(3):663–666
Hu C, Wu C, Yang E, Huang H, Xu D, Hou Y et al (2019) Serum KL-6 is associated with the severity of interstitial lung disease in Chinese patients with polymyositis and dermatomyositis. Clin Rheumatol 38(8):2181–2187
Yanaba K, Hasegawa M, Takehara K, Sato S (2004) Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol 31(6):1112–1120
Elhai M, Hoffmann-Vold AM, Avouac J, Pezet S, Cauvet A, Leblond A et al (2019) Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 71(6):972–982
Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW (2019) Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 21(1):58
Xu L, Yan DR, Zhu SL, Gu J, Bian W, Rong ZH et al (2013) KL-6 regulated the expression of HGF, collagen and myofibroblast differentiation. Eur Rev Med Pharmacol Sci 17(22):3073–3077
Wakamatsu K, Nagata N, Kumazoe H, Oda K, Ishimoto H, Yoshimi M et al (2017) Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig 55(1):16–23
Ishikawa N, Hattori N, Yokoyama A, Kohno N (2012) Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 50(1):3–13
Bonella F, Volpe A, Caramaschi P, Nava C, Ferrari P, Schenk K et al (2011) Surfactant protein D and KL-6 serum levels in systemic sclerosis: correlation with lung and systemic involvement. Sarcoidosis Vasc Diffuse Lung Dis 28(1):27–33
Sokai A, Tanizawa K, Handa T, Kanatani K, Kubo T, Ikezoe K, et al. Importance of serial changes in biomarkers in idiopathic pulmonary fibrosis. ERJ Open Res. 2017;3(3).
Kumánovics G, Péntek M, Bae S, Opris D, Khanna D, Furst DE et al (2017) Assessment of skin involvement in systemic sclerosis. Rheumatology (Oxford) 56(suppl_5):v53–v66
Sánchez-Díez S, Munoz X, Ojanguren I, Romero-Mesones C, Espejo D, Villar A et al (2022) YKL-40 and KL-6 Levels in serum and sputum of patients diagnosed with hypersensitivity pneumonitis. J Allergy Clin Immunol Pract 10(9):2414–2423
Hu Y, Wang LS, ** YP, Du SS, Du YK, He X et al (2017) Serum Krebs von den Lungen-6 level as a diagnostic biomarker for interstitial lung disease in Chinese patients. Clin Respir J 11(3):337–345
Osaka A, Yanagihara K, Yamada Y, Hasegawa H, Inokuchi N, Hayashi T et al (2009) Elevation of serum KL-6 glycoprotein or surfactant protein-D in adult T-cell leukemia with distinct pulmonary complications. Tohoku J Exp Med 218(2):99–105
Acknowledgements
Not applicable
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
Mk gave idea, followed the patients, collected the patients’ data and analyzed them, and wrote the paper. AF put the study design and helped in writing the paper. AS collected the patients; data and analyzed them. NA did the practical part and put the study design.GA collected the patients and helped in writing the paper. SS collected the patients and helped in writing the paper. This manuscript has been read and approved by all the authors; the requirements for authorship have been met
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consents according to Helsinki declaration were taken from patients and control subjects prior to participation in the study that was approved by the Research Ethics Committee of the Institute of the Faculty of Medicine, Benha University MS(13–7-2020).
Consent for publication
Non applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalil, M., Fouda, A.I., Amin, N.A. et al. KL-6 in adult polymyositis and dermatomyositis patients and its correlation with interstitial lung disease. Egypt Rheumatol Rehabil 50, 64 (2023). https://doi.org/10.1186/s43166-023-00206-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00206-9