Abstract
Background
Many viral infections can cause hearing loss due to affection of cochlear hair cells or neurogenic pathway. Although, the damage secondary to viral infections is mainly cochlear affection; auditory brainstem can be affected as well. It was predicted that SARS-COV-2 infection can similarly affect the auditory system. This study aimed to detect affection in auditory system and if present investigate the possible site of lesion (up to the level of the brain stem) in relation to COVID-19 infection.
Methods
This is a case control study, where the study group constituted of thirty adults, diagnosed with COVID-19 at least 2 weeks prior to testing and up to 6 months, without previous auditory complaints pre-COVID-19 or other risk factors that could affect the auditory pathway. Fifteen adult participants that were age and gender matched to the study group with no previous history of covid-19 infection constituted the control group. Audiological evaluations done to all participants were pure-tone and speech audiometry, tympanometry, transient-evoked otoacoustic emission with and without contralateral suppression and auditory brainstem response measurements.
Results
The study group showed significantly worse pure tone thresholds at high frequencies 4 and 8 kHz (p < 0.01), significantly worse transient-evoked otoacoustic emission signal to noise ratio at 2800 Hz and 4000 Hz (p < 0.05) and significantly lower total suppression index (p<0.05). On the other hand, there was no significant difference between both groups in auditory brainstem response wave latencies (p > 0.05).
Conclusion
COVID-19 had subtle effect on cochlear basal turn, and it is shown that the auditory efferent system may also be affected, while the auditory nerve and afferent brainstem pathways seems to be spared. Moreover, the absence of the symptoms of auditory dysfunction postcovid-19 does not guarantee normal auditory functions.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection which emerged in December 2019 in Wuhan, China [1].
Many viral infections can cause hearing loss through affecting cochlear hair cells or neurogenic pathways [2]. Although, the damage caused secondary to viral infections is intracochlear; auditory brainstem can be affected as well [3]. It was predicted that SARS-COV-2 infection can similarly affect the auditory system [4].
Cases of sensorineural hearing loss related to COVID-19 infection have been reported with different degrees of severity in different age groups [5, 6].
The pathogenesis of hearing loss had been described by different researchers. While there had been no full explanations yet, some authors suggested direct infection of the inner ear, while others pointed to possibility of indirect effect on the auditory system. It has also been reported that SARS-CoV-2 can affect the brainstem (part of the central auditory pathway) with vascular compromise, inflammation and neurodegeneration [7].
Various symptoms have been associated with COVID-19; it is believed that hearing loss may be one of the consequences of the infection. However, there are still debate within the literature about its effect on the auditory system. Thus, the current study aimed to first: detect presence of auditory system affection in COVID-19 patients. Second: to estimate the possible site of lesion if present (up to the level of the brain stem) in relation to COVID-19 infection and lastly to study effect of disease variables on presence of auditory dysfunction.
Methods
This is a case control study. The present study was conducted in Audiology clinic. The purpose and method of the study were explained to each participant and informed consent was taken.
The study was comprised of two groups: study and control group.
Thirty subjects with past history of COVID-19 infection constituted the study group.
Patients aged 18-60 years who were diagnosed with the COVID-19 disease by laboratory tests and/or radiological investigations at least 2 weeks prior to testing and up to 6 months were included in the study. Patients with COVID-19 infection more than 6 months prior to testing were excluded as the longer duration will decrease the assumption that COVID-19 is the cause of auditory pathology.
Exclusion criteria included age > 60 years, patients with confirmed neurological disorders or any disorder that can affect the central auditory pathway prior to infection with COVID-19, patients who were previously diagnosed with hearing loss or had complaints suggesting auditory dysfunction prior to infection with COVID-19 and patients with systemic diseases that can affect the peripheral or central auditory pathway or other risk factors for auditory affection including positive family history of hearing loss, noise exposure and ototoxicity.
The study group included cases with single or recurrent infections and different degrees of COVID-19 severity: a. mild disease (no signs of pneumonia and recovered at home) b. moderate degree (presence of clinical or radiographic evidence of lower respiratory tract disease, but with a blood oxygen saturation of 90 percent or higher) and c. severe degree (patients who needed hospitalization on oxygen support) adapted from [8].
Control group constituted of fifteen normal hearing subjects age and gender matched to study group. All subjects did not have any history suggestive of peripheral or central auditory pathway affection or COVID-19 infection.
All patients underwent the following:
-
Full audiological history including presence of auditory complaints as hearing loss, tinnitus, earache, discharge and ear fullness and their onset.
-
COVID-19 history including method of diagnosis, symptoms severity, number of attacks and treatment.
-
Otological Examination.
-
Basic Audiological Evaluation including:
-
Pure tone audiometry was performed using audiometer (AC40 model; Interacoustics, Denmark) in a sound treated room at frequencies 250-8000 Hz for air conduction and 500-4000 Hz for bone conduction.
-
Speech audiometry: speech reception threshold (SRT) using adult Arabic spondee words and speech discrimination scores using adult Arabic Phonetically Balanced monosyllabic words.
-
Acoustic immittancemetry with tympanometer (MADSEN Zodiac model 1096, SA), tympanometry was obtained using 226-Hz probe tone.
-
-
Transient evoked Otoacoustic emission with and without contralateral suppression: to measure outer hair cell function and test function of the medial olivocochlear bundle (efferent auditory pathway) through contralateral suppression of otoacoustic emissions [9].
Transient Evoked Otoacoustic Emission (TEOAE) were elicited on Otodynamics Ltd ILOv6 (United Kingdom) using 80 u linear click stimuli with rate of 50 clicks/s for all subjects (as they all showed normal otoscopy and tympanometry).
To assess effect of contralateral suppression: a contralateral broad band noise (CBBN) (white noise) was generated by the ILO software and presented at 0 signal to noise ratio (SNR) in the contralateral ear at 60 dBSPL.
The response was evaluated in terms of: Response SNR in five frequency bands (1, 1.4, 2, 2.8, and 4 KHz) and were considered present when the signal-to-noise ratio was 6 dB or greater in at least 3 frequencies. In addition to Absolute suppression effect: calculated by subtracting the SNR with CBBN from without CBBN and expressed in dB for the overall response [10].
-
Neuro-otologic Auditory brainstem response (ABR): ABR measurements were made using the device ICS Charter EP 200 equipment (GN Otometrics, Denmark). The test was conducted while the patient was naturally slee** or staying quiet and relaxed on the bed to avoid artifacts.
One channel recording was used with active electrode placed on the forehead, the reference electrode on the ipsilateral mastoid and the ground electrode on the contralateral mastoid.
Auditory Brainstem Response (ABR) was recorded using acoustic clicks of 0.1 msec duration with rarefaction polarity; presented at intensity of 90 dBnHL in each ear separately through insert earphones. The clicks were presented first at low rate of 21.1 p/sec then another recording with higher stimulus rate 71.1 p/sec was obtained to reveal any subtle auditory brain stem pathway abnormalities. At least two recordings were obtained for each stimulus rates. A total number of 1024 sweeps for each recording were differentially amplified and filtered through a band pass filter of 100 to 1500 Hz.
Response was assessed for absolute and interpeak wave latencies, interaural wave V latency difference and latency rate function of wave V through measuring difference in absolute latencies of wave V between low and high-rate stimulation.
Statistical analysis
The collected data was revised, coded, tabulated and introduced to a PC using statistical package for social sciences (IBM SPSS) version 23. The quantitative variables obtained in the study were tested for normal distribution using Kolmogorov–Smirnov tests. Descriptive data were presented according to the type of data. Comparisons between the two groups were analyzed using the independent t-test when the normality condition was met and the Mann–Whitney U test when not. Spearman rank correlation was used to correlate two numerical values that are not normally distributed. The p-value was considered significant as the following: P-value > 0.05: Non-significant (NS), P-value < 0.05: Significant (S), P-value < 0.01: Highly significant (HS).
Results
A total of 45 people were included in the study. The study group consisted of 30 individuals (17 females and 13 males; mean age: 34.8 ± 9.81 years). The control group consisted of 15 individuals (7 females and 8 males; mean age: 32.8 ± 8.28 years).
Most of the study group (19 patient, 63.33%) had mild illness, followed by moderate (7 patients, 23.33%) and only (4 patients, 13.33%) with severe infection. The majority presented with pulmonary symptoms as cough and shortness of breath accompanied with loss of taste and smell in 26 patients (86.7%).
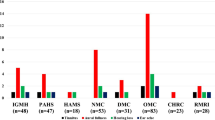
In the study group, the symptoms of auditory dysfunction in the group with COVID-19 were presented in 14 patients (46.7 %), 6 individuals (20 percent) complained of tinnitus including one patient reporting both tinnitus and hearing loss, 6 patients (20%) complained of aural fullness including 1 patient reporting fullness accompanied by earache and diminution of hearing and 2 patients (0.07%) had earache (Fig. 1).
Basic audiological evaluation
All patients in the study group had excellent speech discrimination scores and normal pure tone audiometry (≤25 dBHL) except 2 patients with severe COVID-19 had mild high frequency hearing loss at 8000 Hz only.
A statistically high significant difference was found between both groups at high frequencies only (4 and 8 KHz) (p < 0.01) (Fig. 2).
All subjects had bilateral type (A) tympanograms reflecting normal middle ear pressure.
TEOAE
All subjects achieved pass criteria for TEOAE except in one ear of the study group. TEOAE SNR obtained for each frequency in the control and study groups were compared. A significant difference was found between both groups at 2800 Hz and 4000 Hz frequencies (p < 0.05) as shown in Table 1.
Upon introducing Contra-lateral broad band noise suppression, the total suppression index in the study group mean was 0.29±1.73 and in the control group was 0.97 ±0.89 showing statistically significant difference between both groups (T= -2.032, p value= 0.045).
ABR: All patients had good waveform morphology with no significant difference between both groups in all ABR latency parameters (p > 0.05) as shown in Table 2.
Relation to disease variables
Tests that differed significantly than control were studied further for effect of disease variables (including pure tone audiometry at high frequencies, TEOAE at high frequencies and total suppression index).
In patients with a history of Covid-19, the mean period from Covid-19 diagnosis to the time of testing was 3.85 ±1.81 months. Duration passed from covid-19 infection didn’t show any significant correlations with test results (Table 3).
The majority of patients (18 participant, 60%) had recurrent infections with covid-19. There was no significant effect of covid-19 recurrent infections on audiological dysfunction (p>0.05) except in TEOAE at high frequencies were lower (worse) SNR was observed reaching significance at 2.8 KHz (p<0.01) (Table 4).
Effect of presence of auditory complaints on audiological test findings
Patients with auditory complaints (diminution of hearing, tinnitus, ear fullness, earache) showed no significant difference compared to patients with no complaints (p>0.05) except for pure tone audiometry at 8000 Hz, where complaining patients had significantly worse thresholds (p<0.05).
Discussion
Most of the patients in the study group had no auditory complaints, with only 14 patients (46.7%) complained of some auditory dysfunction started within the first month from infection. Similarly, Freni et al. (2020) reported ear damage in 40% of patients using questionnaires [11].
Bilateral tinnitus and ear fullness were the main audiological complaint, representing 20% each. Similarly, Ozturk et al. (2022) reported that, the most common audiological symptom in the group with COVID-19 were tinnitus [12]. While other studies reported aural fullness as the most common audiological symptom [13, 14].
The predominant reports of aural fullness in COVID-19 could be attributed to Eustachian tube dysfunction which may be triggered by upper respiratory infections and can also be explained by the fact that Angiotensin converting enzyme-2 (ACE-2) is predominantly present in the eustachian tube, thus it is susceptible to infection by SARS-CoV-2 [15]. The presence of tinnitus could be attributed to effect of covid-19 infection on the peripheral and central auditory system in addition to psychological triggers exacerbated by the pandemic such as anxiety and depression, which may initiate or worsen tinnitus [16].
On performing pure tone audiometry, only 2 patients (6.7%) had mild high frequency sensorineural hearing loss at 8000 Hz. This low percentage is consistent with the reported estimated prevalence of hearing loss with COVID-19 infection in the literature which was about 7.6% [17].
On comparing pure tone audiometric thresholds to control group, (Fig. 1) there was a statistically significant (but not clinical) thresholds elevation in post-COVID-19 group at high frequencies (4 and 8 KHz) which is of minor effect as the mean thresholds at each frequency in the study group were within normal range ≤ 25 dB HL. Similarly, several studies revealed elevated hearing thresholds at high audiometric frequencies among COVID-19 patients compared to control groups with mean thresholds within normal range [12, 18, 19]. On the contrary, Degen et al. (2022) didn’t find significant difference in either hearing thresholds in covid-19 group compared to control [20].
This significant difference can be attributed to inflammatory response with COVID-19 where the cytokine release-mediated inflammatory responses can induce inflammations at the cochlea or auditory nerve [17, 21]. In addition to abnormal immune response, with the production of a large number of autoantibodies, cross-reactions of the antibodies to the inner ear antigens can occur leading to accidental damage to the inner ear [22].
Significant difference in TEOAE SNR was noticed at high frequencies (2.8 and 4 KHz) with the study group showing worse SNR (Table 1). These support a possible subclinical auditory dysfunction evidenced by reduced OAEs amplitudes in conjunction with a lack of elevated audiometric thresholds. This agrees with findings from different studies reporting significantly lower OAE amplitudes at high frequencies in COVID-19 patients which can be related to the intrinsic sensitivity of the hair cells in the basal region [20, 23]. The current findings support the view that COVID-19 can cause mild damage to cochlear outer hair cells mainly situated in the basal turn of the cochlea which can be attributed to ischemia from endothelial damage, thrombotic mechanisms and respiratory distress caused by COVID-19 [17, 24].
On performing TEOAE with contralateral suppression, the total suppression index was significantly lower (worse) in the study group compared to the control group. This agrees with Emekci et al. (2022), who reported that patients with COVID-19 had significantly lower DPOAE results with contralateral suppression compared to healthy individuals [25]. Similarly, Basoz et al. (2022) observed that the mean contralateral suppression test with transient otoacoustic emissions was significantly lower (worse) at high frequency in the study group compared to the control group and this indicates the possible effect of covid-19 infection on the efferent auditory pathway [26].
This effect on TEOAE can be attributed to direct viral invasion where the cellular receptor for the SARS-CoV-2 (angiotensin-converting enzyme 2 [ACE-2] receptors) was confirmed to be present in multiple areas along the auditory pathway including the hair cells, spiral ganglion cells and in the stria vascularis, brainstem and central nervous system (CNS) [15]. The virus may be transmitted to the inner ear through cerebrospinal fluid, the nose, and olfactory foramina to the central nervous system; labyrinthine artery to stria vascularis, Eustachian tube to round or oval windows [22]. Additionally, it has been postulated that SARS-COV-2 have neuroinvasive properties and can possibly infiltrates the central nervous system via the blood–brain barrier or via the olfactory pathway leading to neuroinflammation and neuropathies [15].
In the current study, there was no significant difference between the study and control groups as regards low and high repetition rates in ABR waves absolute and interpeak latencies (Table 2). This finding is consistent with a study conducted by Hassani et al. (2021) who stated that there was no significant difference between the study and control groups as regards ABR parameters at high and low repetition rates [27]. Similarly, Dror et al. (2021) and Visram et al. (2023) reported no significant differences in ABR waves between recovered COVID-19 patients and control [28, 29]. In contrast, Dorobisz et al. (2023) demonstrated longer latencies of waves III, V, and time intervals I–III, I–V in post-COVID-19 patients complaining of hearing loss or tinnitus with the majority of his study group had SNHL [22]. It is thought that the normal latencies found in our study indicate no significant effect of covid-19 on neural or afferent auditory brainstem pathways from auditory nerve up to midbrain level in brainstem.
The above-mentioned findings suggest minor effect on cochlear outer hair cells at basal regions, abnormal function of efferent auditory system which can be attributed to direct causes as direct viral invasion to inner ear and CNS or indirect routes including inflammatory response, autoimmune reactions and ischemia.
Relations to disease characteristics
As regards TEOAEs, worse SNR was observed reaching significance at 2.8 KHz in patients with recurrent infections of COVID-19 (Table 4).
No other significant effects were observed in audiological test findings with recurrent infection or duration passed from infection.
Effect of presence of auditory complaints
In the current study, significantly higher (worse) pure tone thresholds at 8000 Hz were found in the group of patients with complaints of auditory dysfunction (Table 5) without significant effect on the other audiological test findings.
Thus, the current study indicated that one attack of covid-19 can be sufficient to induce the observed effect on auditory pathway and that absence of auditory complaints does not guarantee the proper function of the auditory pathway. In addition, test result findings didn’t show improvement or decrement with duration passed from infection, however, longitudinal studies would be needed to confirm this possibility.
Conclusions
COVID-19 had subtle effect on cochlear basal turn, and the auditory efferent system may also be affected, while the nerve and afferent pathways seems to be spared. Moreover, the absence of the symptoms of auditory dysfunction postcovid-19 does not guarantee normal auditory functions.
References
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 324:782–793. https://doi.org/10.1001/jama.2020.12839
Yıldız E (2022) Comparison of pure tone audiometry thresholds and transient evoked otoacoustic emissions (TEOAE) of patients with and without Covid-19 pneumonia. Am J Otolaryngol 43:103377. https://doi.org/10.1016/j.amjoto.2022.103377
Chirakkal P, Al Hail AN, Zada N, Vijayakumar DS (2021) COVID-19 and Tinnitus. Ear Nose Throat J 100:160–162. https://doi.org/10.1177/0145561320974849
Ogier M, Andéol G, Sagui E, Dal Bo G (2020) How to detect and track chronic neurologic sequelae of COVID-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up. Brain Behav Immun Health 5:100081. https://doi.org/10.1016/j.bbih.2020.100081
Soler ZM, Patel ZM, Turner JH, Holbrook EH (2020) A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol 10:814–820. https://doi.org/10.1002/alr.22578
Fancello V, Fancello G, Hatzopoulos S, Bianchini C, Stomeo F, Pelucchi S et al (2022) Sensorineural Hearing Loss Post-COVID-19 Infection: An Update. Audiol Res 12:307–315. https://doi.org/10.3390/audiolres12030032
Yong SJ (2021) Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem Neurosci 12:573–580. https://doi.org/10.1021/acschemneuro.0c00793
World Health Organization (2020) Clinical management of COVID-19 interim guidance. Internet Publication. https://apps.who.int/iris/handle/10665/332196. Accessed 7 Mar 2022
Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A (1990) Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res 43:251–261. https://doi.org/10.1016/0378-5955(90)90232-e
Veuillet E, Collet L, Duclaux R (1991) Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65:724–735. https://doi.org/10.1152/jn.1991.65.3.724
Freni F, Meduri A, Francesco G, Nicastro V, Galletti C, Aragona P et al (2020) Symptomatology in head and neck district in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol 41:102612. https://doi.org/10.1016/j.amjoto.2020.102612
Öztürk B, Kavruk H, Aykul A (2022) Audiological findings in individuals diagnosed with COVID-19. Am J Otolaryngol 43:103428. https://doi.org/10.1016/j.amjoto.2022.103428
Kökoğlu K, Tektaş N, Baktir-Okcesiz FE, Şahin Mİ (2021) Mild and moderate COVID-19 disease does not affect hearing function permanently: a cross-sectional study involving young and middle-aged healthcare givers. Eur Arch Otorhinolaryngol 278:3299–3305. https://doi.org/10.1007/s00405-021-06883-6
Bhatta S, Sharma S, Sharma D, Maharjan L, Bhattachan S, Sah MK et al (2022) Study of Hearing Status in COVID-19 Patients: A Multicentered Review. Indian J Otolaryngol Head Neck Surg 74:3036–3042. https://doi.org/10.1007/s12070-021-02710-w
Almishaal AA, Alrushaidan AA (2022) Short- and Long-Term Self-Reported Audiovestibular Symptoms of SARS-CoV-2 Infection in Hospitalized and Nonhospitalized Patients. Audiol Neurootol 27:297–311. https://doi.org/10.1159/000521963
Daher GS, Nassiri AM, Vanichkachorn G, Carlson ML, Neff BA, Driscoll CLW (2022) New onset tinnitus in the absence of hearing changes following COVID-19 infection. Am J Otolaryngol 43(1):103208. https://doi.org/10.1016/j.amjoto.2021.103208
Gabr T, Kotait M, Moaty AS (2022) Audiovestibular and vaccination complications of COVID-19. Egypt J Otolaryngol 38:105. https://doi.org/10.1186/s43163-022-00290-2
Mustafa MWM (2020) Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol 41:102483. https://doi.org/10.1016/j.amjoto.2020.102483
Tan M, Cengiz DU, Demir İ, Demirel S, Çolak SC, Karakaş O et al (2022) Effects of Covid-19 on the audio-vestibular system. Am J Otolaryngol 43:103173. https://doi.org/10.1016/j.amjoto.2021.103173
Degen CV, Mikuteit M, Niewolik J, Joosten T, Schröder D, Vahldiek K et al (2022) Audiological profile of adult Long COVID patients. Am J Otolaryngol 43:103579. https://doi.org/10.1016/j.amjoto.2022.103579
Kaliyappan K, Chen YC, Krishnan Muthaiah VP (2022) Vestibular Cochlear Manifestations in COVID-19 Cases. Front Neurol 13:850337. https://doi.org/10.3389/fneur.2022.850337
Dorobisz K, Pazdro-Zastawny K, Misiak P, Kruk-Krzemień A, Zatoński T (2023) Sensorineural Hearing Loss in Patients with Long-COVID-19: Objective and Behavioral Audiometric Findings. Infect Drug Resist 16:1931–1939. https://doi.org/10.2147/IDR.S398126
Gedik Ö, Hüsam H, Başöz M, Tas N, Aksoy F (2021) The effect of coronavirus disease 2019 on the hearing system. J Laryngol Otol 135(9):810–814. https://doi.org/10.1017/S0022215121001961
De Luca P, Scarpa A, Ralli M, Tassone D, Simone M, De Campora L et al (2021) Auditory Disturbances and SARS-CoV-2 Infection: Brain Inflammation or Cochlear Affection? Systematic Review and Discussion of Potential Pathogenesis. Front Neurol 12:707207. https://doi.org/10.3389/fneur.2021.707207
Emekci T, Dündar MA, Kirazlı G, Men Kılınç F, Cengiz DU, Karababa E et al (2022) Evaluation of the efferent auditory system in COVID-19 adult patients. Acta Otolaryngol 142:509–514. https://doi.org/10.1080/00016489.2022.2093967
Basoz M, Tas N, Gedik O, Ozdemir S, Aksoy F (2022) Transient otoacoustic emissions with contralateral suppression findings in COVID-19 patients. Egypt J Otolaryngol 38:43. https://doi.org/10.1186/s43163-022-00231-z
Hassani S, Lazem M, Jafari Z (2021) No lasting impact of Covid-19 on the auditory system: a prospective cohort study. J Laryngol Otol 135(12):1063–1068. https://doi.org/10.1017/S002221512100267X
Dror AA, Kassis-Karayanni N, Oved A, Daoud A, Eisenbach N, Mizrachi M et al (2021) Auditory Performance in Recovered SARS-COV-2 Patients. Otol Neurotol 42:666–670. https://doi.org/10.1097/MAO.0000000000003037
Visram AS, Jackson IR, Guest H, Plack CJ, Brij S, Chaudhuri N et al (2023) Pre-registered controlled comparison of auditory function reveals no difference between hospitalised adults with and without COVID-19. Int J Audiol https://doi.org/10.1080/14992027.2023.2213841
Author information
Authors and Affiliations
Contributions
AN contributed to the Conception and design of the work, interpretation, supervision and reviewed the manuscript. RE contributed to title page and declarations, the conception, design of the work, interpretation and reviewed the manuscript. NH contributed to acquisition, data collection and analysis. AE contributed to the conception, design of the work, data interpretation and in the writing of the original draft and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nassar, A.AM., El-Kabarity, R.H., El-Din Hassan, N.N. et al. Evaluation of cochlear and auditory brainstem functions in COVID-19 patients; a case control study. Egypt J Otolaryngol 40, 29 (2024). https://doi.org/10.1186/s43163-024-00580-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-024-00580-x