Abstract
Background
Irrational use of antibiotics in hospitals is one of the main health system problems. It leads to antibiotic resistance, adverse events, treatment failure, total treatment costs, and longer hospital stay. We aim to evaluate clindamycin use in critical care units in our hospital. It is a step to assess and then put strategies to improve the antibiotic use process.
Methods
This is a clindamycin use evaluation retrospective study. It was done in critical care units at Alexandria’s main university hospital. The clinical pharmacists reviewed 99 patients’ prescriptions over the last 4 months, recording patients' demographics, main diagnosis, comorbidities, type of infection, duplication of therapy, dose, the occurrence of diarrhea, serious drug interactions, clindamycin-defined daily dose per 1000 patients’ days), treatment duration and total cost of clindamycin.
Results
A total of 99 patients were included. Clindamycin was prescribed in appropriate indications in 57 patients (57/99 = 57.6%). Prescriptions with inappropriate indications were 42 (42/99 = 42.4%). Duplication of therapy with clindamycin was detected in 32 prescriptions (32.3%). Diarrhea was recorded in 4% of the cases. There were no severe drug interactions with clindamycin. Inappropriate indications were 320 defined Daily Dose (DDD) of total clindamycin consumption (765 DDD) and a cost of 29951.5 LE (42% of total cost). The prescribed dose of clindamycin was correct in all cases.
Conclusion
There is irrational clindamycin use in critical care units in some cases regarding indications and treatment duplication. Although the prescribed doses were correct. Clindamycin misuse increased total consumption and cost.
Trial registration number
NCT05223400 on 2 February 2022.
Similar content being viewed by others
Background
Irrational use of antibiotics in hospitals is one of the main health system problems. It may include inappropriate indication, dose, treatment course duration, and other drug management processes. It leads to antibiotic resistance, adverse events, treatment failure, total treatment costs, and longer hospital stays. World health organization (WHO) and centers for disease control and preventions (CDC) encourage hospitals to establish their antibiotic stewardship programs to apply strict regulations of antibiotics use [1,2,3].
Medication use evaluation studies are performance improvement tools. They are used to evaluate and improve one or more medication management processes. Medication use evaluation studies can be retrospective, prospective, or cross-sectional. The most used type is the retrospective one [4, 5].
Clindamycin is a lacosamide antibiotic. It is approved from the Federal and Drug Administration Agency for the treatment of community-acquired aspiration pneumonia, skin, and soft tissue infections, osteomyelitis, septic arthritis, toxic shock syndrome, pelvic infection, bacterial vaginosis, toxoplasma gondii encephalitis, malaria, and anthrax. It is active against staphylococci, streptococci, pneumococci, most gram-positive and gram-negative anaerobes, and chlamydia [6].
Medications consumption registries in critical care units showed that clindamycin use is increasing. In some cases, the clinical pharmacists' reports also refer to inappropriate prescribing practices.
Methods
Aim
We hypothesized that there is irrational use of clindamycin. So, we needed a clindamycin use evaluation study.
Design
This is a retrospective observational study in critical care units at Alexandria Main University Hospital.
There is no need for informed consent as it is a performance improvement study.
After registration of the protocol in the hospital medical ethics committee on 16 /9/2021 with serial number 0305292 and in clinical trials.gov, two critical care clinical pharmacists started collecting data. They reviewed prescriptions including empirical intravenous clindamycin therapy during the last 4 months to collect the sample size of 99 patients. They documented patients' demographic characteristics, main diagnosis, comorbidities, type of infection, duplication of therapy, dose, the occurrence of diarrhea as the main adverse event, serious drug interactions, clindamycin-defined daily dose, clindamycin-defined daily dose per 1000 patients’ days (DDD/ 1000 patients’ days), treatment duration and total cost of clindamycin.
Defined daily dose (DDD)
The assumed average maintenance dose per day for a drug used for its main indication in adults.
The DDDs are allocated to drugs by the WHO Collaborating Centre in Oslo, working in close association with the WHO International Working Group on Drug Statistics Methodology [7]
DDD/ 1000 patients’ days is another tool to allow antibiotic consumption comparison through different healthcare settings or different situations [8].
The sample size (n) is calculated according to the formula:
Where: z = 1.96 for a confidence level (α) of 95%, p = expected proportion of 50% as a role of thumb (expressed as a decimal), N = population size(number of admitted patients 11*12 months), e = margin of error.
n = 384.16 / 3.9103 = 98.243
n ≈ 99.
The sample size (with finite population correction) is equal to 99 patients.
Descriptive statistics
All categorical variables were presented as counts and percentages and all numerical variables were presented by median and interquartile range as they are not normally distributed as shown after performing the Shapiro test of normality.
Inferential statistics
Comparative statistics were performed by Fischer's exact test to compare two independent proportions or the Wilcoxon rank sum test to compare two independent medians the p-value is considered significant if it was < 0.05.
Inclusion criteria
-
Adults ≥ 18 years old.
-
Patients started clindamycin empirically in critical care units.
-
Patients started clindamycin empirically in other departments and the physicians approved to continue in critical care.
Exclusion criteria
Children (less than 18 years old)
-
Patients started clindamycin as a definitive treatment (based on microbiological cultures results).
-
For patients who started clindamycin as a definitive treatment after an initial period of clindamycin empirical course, the calculation of DDD and total cost of clindamycin will be stopped before the definitive treatment period.
Results
A total number of 99 prescriptions including clindamycin as an empirical therapy were reviewed (Table 1).
The researcher recorded the primary patients’ diagnosis and resulted in 62% of cases had cerebral or neurological disorders (Figs. 1 and 2). Patients’ comorbidities are mainly cardiovascular or metabolic disorders.
After documentation and specification of the infection types and referring to IDSA guidelines, prescriptions were divided into prescriptions including appropriate clindamycin indications or those with inappropriate indications (Table 2).
The most common appropriate indication was community acquired pneumonia 77% (44/57) while ventilator associated pneumonia represents the highest percentage of inappropriate indications 73.8% (31/42) (Tables 3 and 4).
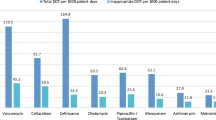
Inappropriate indications increased clindamycin consumption. It was illustrated using treatment duration by days, defined daily dose calculation and total cost.
Discussion
The calculated sample size is 99 patients. All cases fulfilling the inclusion criteria during the whole 4 months are collected during data collection. The results show that the top three main diagnoses on admission are stroke, traumatic or metabolic brain insult, and toxicological causes (incidence rate 40.4%,21.2%, and 13.1%).
Most cases suffer from hypertension, diabetes mellitus, and atrial fibrillation (38.3,27.2, and 13.1%). All of them are risk factors for stroke or metabolic brain insults.
The two most common types of infection upon which clindamycin is prescribed are community-acquired and ventilator-associated aspiration pneumonia (44 and 31 cases respectively). This is explained by the main diagnosis of most cases (stroke, brain insult, and toxicity). All these cases have a risk of disturbing levels of consciousness, aspiration, and the need for mechanical ventilation.
Referring to IDSA guidelines, physicians prescribed clindamycin in appropriate indications in 57 patients (57/99 = 57.6%) to treat community-acquired aspiration pneumonia, skin, soft tissue infection, and lung abscess. Prescriptions with inappropriate indications are 42 (42/99 = 42.4%).
Clindamycin is misused to treat ventilator-associated pneumonia, aspiration pneumonitis, meningitis, and intraabdominal infection.
In CNS and intraabdominal infections, clindamycin cannot cross the blood–brain barrier to be a treatment option, and it cannot be used as sole therapy in Intra-abdominal infections. While aspiration pneumonitis is inflammation with no evidence of infection [9]. The causative organisms in community-acquired aspiration are streptococci and anaerobes, whereas ventilator-associated pneumonia, usually are gram-negative bacilli with an unclear role of anaerobes [10].
The prescriptions with duplication of therapy with clindamycin were 32 (32.3% of the total prescriptions). Duplication was considered because Meropenem, Imipenem-Cilastatin, and piperacillin-tazobactam have good anaerobic activity. No need for clindamycin as an add-on therapy to cover anaerobes. Regarding infections with gram-positive organisms, there is no benefit of using double coverage with clindamycin with either vancomycin or teicoplanin.
The most common adverse effects of clindamycin are diarrhea, clostridium defficile (CD) infection, and colitis. The occurrence of diarrhea was recorded in 4 cases (4% of the total number of cases confirming CD infection was not possible at the time of the study [9].
In the study, there is no prescription for severe interactions with clindamycin.
Defined daily dose DDD is a parameter recommended by WHO as a tool for antibiotics consumption.
Defined daily dose (DDD)
The assumed average maintenance dose per day for a drug used for its main indication in adults.
The DDDs are allocated to drugs by the WHO Collaborating Centre in Oslo, working in close association with the WHO International Working Group on Drug Statistics Methodology [7, 8].
DDD/ 1000 patients’ days is another tool to allow antibiotic consumption comparison through different healthcare settings or different situations.
- The study calculates both Clindamycin DDD and DDD /1000 patients’ days. The total number of days, bed capacity in critical care units, and average capacity index during this period (4 months) are used to calculate DDD/ 1000 patients’ days.
- The prescribed dose in all cases was 600 mg IV q 8 h, which is the correct dose.
Kamali et al. is the most similar study. It assessed clindamycin use all over the hospital. 607 patients receiving clindamycin during 15 months of study were evaluated. The study reported indication, dose, and duration of clindamycin which was appropriate in 583 (96%), 277 (47.5%), and 208 (35.7%) patients, respectively. The incorrect indications of clindamycin in the study were urinary tract infection and gastrointestinal bleeding [11].
Bekeke et al. study evaluated the rationality of antibiotics used in 248 patients Pediatric ward of Shambu General Hospital in which 600 antibiotics of different classes were prescribed. Dose, frequency, and duration were appropriate in 496 patients (88.57%), 42(75.35%), and 518(92.5%) respectively. Only one antibiotic(chloramphenicol) was contraindicated. Of the total 248 pediatric patients, 76 (30.65%) of them were prescribed drugs, which had possible potential interaction [12].
Kabbara et al. chose to evaluate fluroquinolones as a group of the most prescribed antibiotics in their hospitals. The evaluation included 118 patients. The results showed that the indications, doses, duration of treatment were appropriate in 93.2%, 74.6%, and 57.6% of patients respectively. Only 57% of patients with renal impairment received accurate adjusted doses. The study also investigated the occurrence of dysglycemia as a common adverse event. Hyperglycemia was more common than hypoglycemia and the highest incidence was noticed with levofloxacin use [13].
In a study evaluating 3408 patients in 18 different hospitals in Egypt. 59% of patients were receiving one or more antibiotic agents at the time of survey completion. This is substantially higher than the prevalence of antibiotic use reported in similar studies performed in Europe and the United States of America. Although the prevalence of antibiotic use was quite variable among participating hospitals, ranging from 32.9%–91.7%, all the participating hospitals exceeded the 29% prevalence reported in the 2009 ESAC Survey conducted in 172 hospitals representing 29 European countries [14].
Strengths
As far as we know this is the first clindamycin use evaluation study in Egypt and one of the few clindamycin evaluation studies worldwide. The study evaluates many parameters regarding clindamycin use as the indications, doses, duration, treatment duplication, the occurrence of diarrhea as the main adverse event, serious drug interactions, clindamycin-defined daily dose per 1000 patients’ days, and total cost of clindamycin.
It represents an important step toward implementing an antibiotic stewardship policy in our hospital.
It puts a spotlight on medication use evaluation studies. It attracts the attention of Egyptian healthcare team members and researchers to perform this type of study.
Limitations
The study has a small sample size. It was conducted only in critical care units.
Conclusion
The study detected that irrational clindamycin use in critical care units was related to indications and spectrum of activity. However, the prescribed doses were correct. As a result, this misuse increased total clindamycin consumption and cost.
-We recommend that an Antibiotic stewardship program should be implemented in our hospital as soon as possible.
-In the current situation some strategies should be continued or applied as post-prescription review, preauthorization, and antibiotics awareness lectures to healthcare team members.
- Similar studies should be done in all hospital departments.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DDD:
-
Defined Daily Dose
- WHO:
-
World health organization
- CDC:
-
Centers for disease control and preventions
- IDSA:
-
Infectious disease society of America
- IQR:
-
Interquartile range
- CD:
-
Clostridium defficile
References
Core elements of hospital antibiotic stewardship programs. Centers For Disease Control And Prevention (2019). Available via https://www.cdc.gov/antibiotic-use/core-elements/index.html
Antimicrobial stewardship programmes in health-care facilities in low- andmiddle-income countries. A practical toolkit. World Health Organization. Available via https://www.who.int/publications/i/item/9789241515481 Accessed 22 Oct 2019
Ha DR, Haste NM, Gluckstein DP (2017) The role of antibiotic stewardship in promoting appropriate antibiotic use. Am J Lifestyle Med. 376(4):383–13. https://doi.org/10.1177/1559827617700824
Afanasjeva J, Burk M, Cunningham FF, Fanikos J, Gabay M, Hayes GJ, Masters PL, Rodriguez R, Sinnett MJ (2021) ASHP guidelines on medication-use evaluation. Am J Health Syst Pharm. 168(2):175–78. https://doi.org/10.1093/ajhp/zxaa393. PMID: 33399190
Ruby G, Arvind K, Abrar AZ, Amit S, Ranjeet K (2023) The role of drug utilization evaluation in medical sciences. Glob Health J. 3(1):8–7. https://doi.org/10.1016/j.glohj.2023.02.002
Clindamycin: An overview (2023). http://www.uptodate.com.Aceested 11 Jan 2023
ATC/DDD index (2023). World Health Organization. Available via https://www.whocc.no/atc_ddd_index/. Accessed 23 Jan 2023
Hutchinson JM, Patrick DM, Marra F, Ng H, Bowie WR, Heule L, Muscat M, Monnet DL (2004) Measurement of antibiotic consumption A practical guide to the use of the anatomical therapeutic chemical classification and defined daily dose system methodology in Canada. Can J Infect Dis. 29(1):35–15. https://doi.org/10.1155/2004/389092. PMID: 18159441; PMCID: PMC2094921
Murphy PB, Bistas KG, Le JK (2023). Clindamycin. Treasure Island (FL): Stat Pearls Publishing. Available via https://www.ncbi.nlm.nih.gov/books/NBK519574. Accessed 3 Aug 2023
Mandell LA, Niederman MS (2019) Aspiration pneumonia. N Engl J Med. 651(7):663–380. https://doi.org/10.1056/NEJMra1714562. PMID: 30763196
Ala S, Kamali A, Avan R (2020) Clindamycin stewardship: An opportunity for hospitalized patients in Razi hospital, Rasht. Iran. J. Rep. Pharm. Sci. 73(1):78–9. Available via: https://www.sid.ir/en/journal/ViewPaper.aspx?id=848186.
Firomsa B, Kumera B, Dinka D (2019) Retrospective drug use evaluation of antibiotics in pediatric ward of Shambu general hospital, Oromia region, West Ethiopia. Int J Mod Pharm Res 31(2):41–3
Kabbara W, Ramadan W, Rahbany P, Al-Natour S (2015) Evaluation of the appropriate use of commonly prescribed fluoroquinolones and the risk of dysglycemia. Ther Clin Risk Manag. 639:647–11. https://doi.org/10.2147/TCRM.S81280
Talaat M, Saied T, Kandeel A, El-Ata GA, El-Kholy A, Hafez S, Osman A, Razik MA, Ismail G, El-Masry S, Galal R, Yehia M, Amer A, Calfee DP (2014) A point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics (Basel) 450(3):60–3. https://doi.org/10.3390/antibiotics3030450. PMID: 27025755; PMCID: PMC4790372
Acknowledgements
We would like to thank MARS-Medical Agency for Research and statistics for the biostatistics analysis.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
HS got the study idea, participated in data collection, reviewed other steps with the authors, reviewed the manuscript phrasing, arrangement and submitted the manuscript. BM participated in data collection and manuscript writing. MN Prepared figures and participated in data arrangement and manuscript writing. ME directed the authors and revised the final manuscript form. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaker, H.O., Naguib, M., Abdelaziz, B.M. et al. Clindamycin use evaluation retrospective observational study in critical care units in Alexandria Main University Hospital. Egypt J Intern Med 36, 33 (2024). https://doi.org/10.1186/s43162-024-00300-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00300-0