Abstract
Introduction
Hepatitis E virus (HEV) is one of the leading causes of acute viral hepatitis. There are thought to be 20 million infections per year in poorer nations with inadequate sanitation. In Egypt, awareness about the possible hazards linked to HEV infection is limited due to low socioeconomic and educational levels. Only a small number of sequences have been characterized, making HEV study in Egypt constrained. Numerous factors may have contributed to this neglect. Various extra-hepatic symptoms of HEV infection include neurological problems are recognized. Many European nations have implemented regular HEV monitoring, or targeted screening of blood provided by patients at greater risk to stop the spread of HEV by transfusion.
Aim
Assess the prevalence of HEV infection in asymptomatic blood donors. Increasing awareness about HEV testing in patients with some unexplained neurological disorders.
Methods
Cross-sectional study involving 550 patients: 500 apparently healthy blood donors and 50 patients with some neurological disorders. All subjects were tested for serological markers (IgG and IgM) for HEV using ELISA technique in addition to HEV RNA PCR testing for seropositive patients.
Results
Five hundred asymptomatic blood donors (370 males and 130 females), ages ranging from 20 to 50 years (median 33), 22.6% of them tested positive for HEV (IgG and IgM) of which 2 subjects only had positive HEV RNA PCR testing. In the second group 50 patients (26 males and 24 females) with various unexplained neurological disorders. Liver functions were within normal or showed only a mild increase. Forty-four percent of the patients had positive serology for HEV, with 6 patients testing positive for HEV RNA on PCR.
Conclusion
No need for mass screening for HEV serology among blood donors. HEV infection needs to be considered in patients with unexplained neurological disorders even if the liver functions are not markedly elevated.
Similar content being viewed by others
Introduction
Hepatitis E virus (HEV) is a significant contributor to acute viral hepatitis on a global scale. Each year, roughly 20 million of HEV infections are recorded resulting in approximately 3.3 million hepatitis E cases with symptoms. The World Health Organisation (WHO) estimated that hepatitis E was responsible for 44,000 fatalities in 2015, representing 3.3% of deaths that were caused by viral hepatitis [1]. Enhanced regular testing and preventive actions were advised as a result of a recent meta-analysis and systematic review, which revealed that 15–110 million people either currently or recently have been diagnosed with HEV infection [2]. HEV study has grown significantly over the past ten years, and as a result, our knowledge of HEV epidemiology has transformed from that of a pathogen transmitted through water causing acute infections in underdeveloped countries to that of a worldwide disease with persistent, extra-hepatic, and zoonotic manifestations [3,4,5].
Infection with HEV is linked to a variety of extra-hepatic symptoms, including neurological conditions that are usually described as Guillian-Barre syndrome (GBS) [6]. In India, emerged the first case of GBS linked to HEV [7]. A growing number of cases has lately been identified. There is no distinction between statistics both in industrialized and poor nations. This invalidates the belief that HEV-associated GBS is widespread in such unhygienic settings [6].
Hepatitis E is an endemic virus that is especially prevalent in children and results in severe self-limited hepatitis in Egypt [8]. Based on previous research, those suffering from acute hepatitis have HEV incidences as much as 42% [9].
Due to their poor socioeconomic class and level of education, Egyptians were unaware of any possible risk factors related to HEV infection. HEV genotype 1 subtype 3 became prevalent in rural Egyptian areas as a consequence. Fecal–oral, vehicle-borne, water-borne, zoonotic food-borne, blood-borne via intravenous blood donations, and perinatal are some of the ways that HEV can spread through [10].
In Egypt, there has been little research on HEV, and very few variants have been identified [10]. Despite all of the previous alarms, such negligence may have occurred for a number of reasons: (a) studies conducted on HEV in Egypt between 2000 and 2006 demonstrated that the illness is self-sustaining and that the vast majority of acute hepatitis E (AHE) patients are either asymptomatic or infected sub clinically [3, 11]. (b) A weakened strain of HEV-1 is circulating, which explains the low death rate according to the findings of one significant prospective research project conducted in two Egyptian governorates between 2006 and 2008 on HEV-associated AVH. HEV-associated AVH is not particularly frequent [12]. (c) Due to financial constraints, the majority of the funding that was available in Egypt was used to study other extremely prevalent hepatotropic viruses, such as HCV and HBV [13].
Due to the considerable prevalence of HEV viral load in blood donors, many European countries have implemented baseline HEV testing or targeted screening of blood donated by individuals at increased risk with the objective of avoiding spreading of HEV by transfusion [14]. Various studies have demonstrated that the HEV genes found in plasma of both donors and recipients are the same, supporting the hypothesis that HEV can be transmitted by transfusion [15]. Notably in patients with no symptoms, there is an increasing worry that transfusion-transmitted HEV could constitute an imminent threat to public health. Nearly 50% of recipients of HEV-contaminated blood products contracted the virus, despite the fact that the majority of blood donors at that time appeared symptom-free when they provided these blood products [16]. As the demand for such tests has acquired global interest, blood donors from various nations are getting checked for HEV antibodies [15].
Clinicians may think about and support individualized testing since some recipients, such as those who received transplants, individuals who have hematological cancers, and those with long-term liver disease, have a higher risk of complications. In the UK, focused screening started in 2016. However, it was shown that inventory management, inaccuracies and the original restricted definition of vulnerable individuals led to the conclusion that nationwide screening could be carried out at no extra expense as a result of handling inventories and inaccuracy concerns [17].
It might be required to make sure HEV is excluded if supplied blood or its components will be used by susceptible recipients such pregnant women, those with cancer who are receiving chemotherapy, persons harboring the human immunodeficiency virus, and those undergoing solid organ transplants. Because of this, nucleic acid analysis is the best method for identifying HEV in the blood supplied. Blood donation centers must, however, weigh the profitability and risk-based decisions rationale of performing extra pathogen tests [18].
The likelihood of transfusion-transmitted HEV infection appears to be affected by the blood donor, virus load, and ultimate plasma volume. Several industrialized nations have implemented steps to increase the safety of blood as a consequence of HEV epidemiology [19].
In this study, asymptomatic blood donors’ sera at Alexandria University Hospitals Blood Bank were tested to evaluate if blood transfusion is an important mode of HEV transmission and if it necessitates screening for HEV infection in donors’ sera. A correlation has also been found between the presence of anti-HEV antibodies in the serums of some neurological patients admitted to Alexandria University Hospitals and their clinical findings.
Patients and methods
This cross-sectional study was carried out in Alexandria University Hospitals. The study population included 500 randomly selected blood donors in addition to 50 patients with neurological disorders. Acutely ill patients, known cases of hepatitis B, C, or HIV viral infection were excluded. Moreover those with clear neurological disorder secondary to other neurologically identified syndrome were excluded from the study population. The study was conducted between January 2019 and March 2020.
All the study population was tested for HEV IgG and HEV IgM in their sera using the ELISA technique. Seropositive individuals were tested with RNA PCR for HEV. Neurologically-affected patients were diagnosed by expert neurologists with the following diagnoses: Guillian-Barre syndrome (GBS), myelitis/myelopathy, myasthenia gravis (MG), acute demyelinating encephalomyelitis (ADEM), myeloencephalitis, chronic inflammatory demyelinating polyneuropathy (CIDP), and encephalitis.
Using two different kits, the in vitro enzyme immunoassay (ELISA) approach for the identification of HEV serology was employed to find anti-HEV antibodies present in human plasma or serum [20]. Each was exclusive to a particular class of antibodies; one was meant for IgG antibodies and the other for IgM antibodies. (Abia HEV IgG and Abia HEV IgM of AB Diagnostic Systems GmbH, Berlin, Germany).
The testing process was done according to the standard operating procedure supplied by the kit manufacturer. Serum samples (550 samples) were stored at – 20 °C after collection and separation until the time of processing.
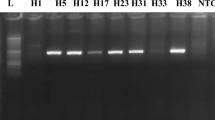
RNA was extracted from frozen samples that had been kept in storage using QIAamp viral RNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer guide supplied with the kit. Using a UV spectrophotometer at 260–280 wavelength, the extracted RNA was quantified and quantitated to evaluate the quality of successfully purified RNA. RNA extraction was followed by QRT-PCR. All of the chemicals were bought from Applied Biosystems (Foster City, CA, USA).
Results
The study population was divided into two groups: group (I) randomly selected blood donors, group (II) patients with neurological disorders.
The blood donor group included 500 individuals with the mean age of the participants being 33.6 ± 7.1 years. Seventy-four percent were males while 26% were females. In the group under study, there was no discernible difference between the genders (p value was 0.63).
Serology laboratory assessment for HEV (IgG and IgM) to group (I) population revealed that 113 donors (22.6%) had IgG positive test results for HEV, while only 4 donors (0.8%) were HEV IgM positive. Regarding the findings of the Hepatitis E virus infection serology, there was no noticeable distinction between the male and female groups (i.e., p value was 0.260). Further analysis of HEV IgM results, we found that four persons had a positive HEV IgM. HEV PCR was positive in the sera of two donors only (Table 1).
Analysis of the distribution of HEV IgG in group I according to the studied population age showed that it is the highest affected age group was those aged 40–50 years old. (31.3% of the IgG seropositive donors) (p value was 0.034).
Regarding the group presented with neurological disorders (II) 50 patients with a mean age of 34.8 ± 12.5 years. Fifty-two percent of patients were males while 48% were females.
We noticed that the most common presenting diagnosis in group II was Guillain–Barre Syndrome (32.0%), followed by myelitis/myelopathy and myasthenia gravis (22.0% each). While only 4% of the patients were diagnosed with encephalitis (Table 2). It was noticed that Guillain–Barre syndrome was more common in males while myelitis/myelopathy was more common in females.
Routine laboratory investigations and liver function tests for group II showed no significant abnormalities in the routine laboratory results with only mild elevation of liver function tests, i.e., ALT and AST (Table 3).
Forty four percent of patients had IgG-positive test results for HEV, while only 4% were IgM-positive. Regarding the HEV IgG result, the percent of seropositive males was 61.5% while the percent of seropositive females was 50%. For the HEV IgM results only four patients tested positive. Four patients had positive results for HEV RNA PCR testing (Table 4).
Comparing patients with Guillain–Barre syndrome, myelitis/myelopathy, myeloencephalitis, encephalitis, myasthenia gravis, chronic inflammatory demyelinating polyneuropathy (CIPD), and acute demyelinating encephalomyelitis (ADEM) on one hand and seropositivity to HEV on the other, p values were 0.525, 0.576, 1.00, 0.306, 0.576, 0.121, respectively. However, among patients suffering from myelitis/myelopathy, 36.4% were diagnosed with HEV infection, compared to 10.7% of those who are seronegative. Statistically significant differences were detected with a p value of 0.042 (Table 5).
HEV seropositivity was detected in 44% of patients with neurological disorders compared with 22.4% of subjects without neurological disorders. The correlation was statistically significant with a p value of 0.001 (Table 6).
Statistical analysis of the data
The Statistical Package of Social Sciences (SPSS) (version 28) was used to analyze the results. The normality of the data was tested using the Kolmogorov–Smirnov test. Qualitative data are described as numbers and percentages. Comparison between qualitative data was done using chi-square or Fisher exact test as appropriate. Numerical variables were presented as mean and standard deviation (SD). To compare the two groups for numerical data, the Mann–Whitney test or t test was used. A P ≤ 0.05 was considered significant.
Discussion
The WHO lists Egypt as having moderate to significant prevalence for several enteric viruses. The significantly elevated percentages of various enteric virus diseases, including hepatitis A and E, human rotaviruses, human noroviruses, human astroviruses, and human adenoviruses, among the Egyptian population serve as an indicator of this [13].
Our study was carried out on 550 subjects classified into two groups: group I consisted of 500 persons who are healthy blood donors while group II included 50 patients with various neurological diseases admitted to Alexandria University Hospitals.
Regarding blood donor group (I), we found that there was no statistical difference between the study population regarding age and gender as the patients were randomly selected. Moreover, no difference among them regarding routine pre-donation checks being properly selected according to blood bank policy.
In our study, 22.6% of blood donors had HEV IgG positive test results. Regarding the results of the serology for hepatitis E virus infection, there was no discernible difference between the male and female groups.
Our results are in agreement with a systematic review and meta-analysis of the seroprevalence of HEV infections in Middle Eastern nations. It demonstrated that the overall combined seroprevalence of HEV infection in Middle Eastern countries was 21.3% and 11.8%, respectively, in the fixed-effect and random-effect models [21].
That meta-analysis by Qashqari FS revealed that among all countries, Egypt had the greatest seroprevalence of HEV infections. HEV infection an undertreated disease in Egypt. HEV screening is not routinely employed in Egyptian hospitals to diagnose patients with probable hepatitis [13].
Our findings contradict those of Stoszek (2006) [22] who demonstrated that Egyptians had one of the highest global seroprevalences of anti-HEV IgG, reaching up to 84.3%. Numerous risk factors were discovered, including poor private hygiene and exposure to cats. However, there were no obvious indications of acute hepatitis or jaundice, which he believed might be a consequence of early-life HEV infections that led to lifelong immunity and altered subsequent reactions to exposure. Moreover, the It was speculated that the major HEV strains in Egypt were less dangerous than those in southern Asia [11].
These reported discrepancies in HEV seroprevalence might be explained by different test procedures, geographical regions, study population size, surveillance period, and other considerations.
This overall prevalence percentage agrees with the results of studies done showing HEV infection prevalence in Egypt, although no recent studies revealed the magnitude of the problem in Alexandria as a non-rural area in Egypt.
In our study, it was found that the most affected age group was the 40–50 years old personnel representing 31.3% of subjects while the least affected group was the age group 20–30 years old representing less than 18.2%.
A study was carried out in Egypt in 1996 by Amer AF et al. to ascertain the prevalence of HEV antibodies in teenagers. The prevalence rate was determined to be 38.9% in 95 healthy adolescent females. 15.8% of participants came from semi-urban settlements, while 84.2% of participants lived in Alexandria Governorate. Participants who were between the ages of 20 and 30 were shown to have a higher prevalence of anti-HEV antibodies than those who were under the age of 10. The majority of people who had serological evidence of jaundice disputed having a HEV infection, proving that the infection was latent. According to that report, HEV is endemic in Alexandria, Egypt [23]. This may be a reflection of increased awareness about health and hand hygiene in addition to environmental sanitation.
Since most HEV infections are asymptomatic, the risk of contaminated blood donors who are undiagnosed is increased [24]. People who donate blood have been found to have high seropositivity rates for anti-HEV IgG, with significant demographic variation [25].
Another study done in Egypt by Ibrahim et al. 2011 on apparently healthy donors with results in agreement with our study showed that 0.45% of samples (i.e., three subjects) had a positive HEV IgM results; two of them were HEV RNA PCR positive. That research concluded that the potential danger of transmission may be limited and that the examined blood donors have a small incidence of persistent subclinical HEV infection [26].
In some studies, the likelihood of transfusion-transmission based on the level of viremia was examined. Given that HEV normally does not cause serious disease, a research done on Australian blood donors assessed the risk to be less than 1 in 35 million, which was evaluated as a tolerable risk [27].
However, it has been shown that in the UK, where individualized testing was first implemented in 2016, the inventory management, error risk, and the initial restrictive definition of at-risk patients have led to the suggestion that nationwide screening may be carried out at no additional expense [28]. As a result, effective nucleic acid analysis for HEV in blood from donors is ideal, while blood banks must weigh the cost-effectiveness and risk-based rationale of any extra pathogen testing of blood supplies.
Regarding the group presented with neurological disorders (II) 50 patients. Twenty-six patients (52%) were males while twenty-four were females (48%).
It has recently become more widely recognized that HEV infection can have neurologic symptoms. Woolson et al. 2014 and Kamar et al. 2011 found in some retrospective studies that 5.5–7.5% of patients with HEV infection experience neurological symptoms [29, 30].
In our study, we noticed that the most common presenting diagnosis in group II was Guillain–Barre syndrome (32.0%), followed by myelitis/myelopathy and myasthenia gravis (22.0% each). While only 4% of the patients were diagnosed with encephalitis.
Our results are consistent with Jha 2021 summary of neurological symptoms with HEV infection, which showed that meningoencephalitis (4%) in addition to disorders of the nerve plexus and root, e.g., neuralgic amyotrophy (39%) and GBS (37%) were the most frequently reported neurological conditions associated with HEV [31].
This is in concordance with Lhomme’s findings from 2021, who discovered that meningoencephalitis and NA are the commonest HEV-associated neurological illnesses [32].
Routine laboratory investigations and liver function tests for group II showed no significant abnormalities in the routine laboratory results with only mild elevation of liver function tests, i.e., ALT and AST. This is in agreement with Jha 2021 where Almost all patients (88%) had normal levels of bilirubin. The levels of alanine aminotransferase (ALT) were highly varied (ranged between 16 and 4502 IU/L with a median of 345.5 IU/L). In the sera of 58% of patients, HEV RNA was identified [31].
A study in Denmark showed that an HEV-related illness should be taken into consideration if there are certain non-liver symptoms, particularly neurological ones such Guillain-Barré syndrome or neurologic amtotrophy with an unexplained mild to moderate elevation in liver enzymes [33].
In disagreement with the above, five cases, predominantly from Europe, of peripheral neuropathy and small fiber neuropathy were recorded. Patients presented with sensory and/or motor neurological manifestations. All patients, except one, did not have jaundice. ALT ranged between 30 and 1606 IU/L with a median of 285 IU/L. In the serum of all five patients, HEV RNA was detected [31].
We found that 22 (44%) patients had IgG-positive test results for HEV, while only 4% were IgM-positive. Regarding HEV IgM testing, only four patients tested positive and all of them had positive HEV RNA PCR test results.
We found that in patients suffering from myelitis/myelopathy, 36.4% were diagnosed with HEV infection, compared to 10.7% of those who are seronegative. Statistically significant differences were detected with a p value of 0.042.
HEV seropositivity was detected in 44% of patients with neurological disorders compared with 22.4% of subjects without neurological disorders. The correlation was statistically significant with a p value of 0.001. In the context of our findings of a strong correlation between HEV infection and some neurological disorders, we recommend screening for HEV infection in patients with unexplained neurological disorders even if the liver functions are not markedly elevated.
The current study is constrained in various ways. There were no controls, the sample size was small, and it included a variety of neurological disorders with different etiologies. Based on that, it was difficult to analyze possible confounding variables. These restrictions should be kept in mind while interpreting our results.
Conclusion
It is not convenient to routinely screen asymptomatic blood donors owing to the low incidence in the studied blood donor population. On the contrary, it is wise to consider HEV subclinical infection in patients presenting with unexplained neurological disorders even though the biochemical tests are normal. However, we think that the preliminary results from the current study warrant further investigation, including case–control studies of HEV infection and neurological illness in specific populations to determine the significance and the details of the association between HEV infection and the injury to the neurological system.
Availability of data and materials
All data and materials are available and can be provided upon request.
References
World Health Organisation (WHO) (2022).Hepatitis E.WHO, Geneva: Switzerlandl; https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Accessed 4 June 2022.
Li P, Liu J, Li Y, Su J, Ma Z, Bramer WM et al (2020) The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis. Liver Int 40(7):1516–1528
El-Mokhtar MA, Othman ER, Khashbah MY, Ismael A, Ghaliony MA, Seddik MI, Sayed IM (2020) Evidence of the extrahepatic replication of hepatitis E virus in human endometrial stromal cells. Pathogens 9(4):295
Sayed IM, Seddik MI, Gaber MA, Saber SH, Mandour SA, El-Mokhtar MA (2020) Replication of hepatitis E virus (HEV) in primary human-derived monocytes and macrophages in vitro. Vaccines (Basel) 8(2):239
El-Mokhtar MA, Seddik MI, Osman A, Adel S, Abdel Aziz EM, Mandour SA, Mohammed N, Zarzour MA, Abdel-Wahid L et al (2020) Hepatitis E virus mediates renal injury via the interaction between the immune cells and renal epithelium. Vaccines (Basel) 8(3):454
Liu H, Ma Y (2020) Hepatitis E virus-associated Guillain-Barre syndrome: revision of the literature. Brain Behav 10(1):e01496
Sood A, Midha V, Sood N (2000) Guillain-Barré syndrome with acute hepatitis E. Am J Gastroenterol 95(12):3667–3668
Hasan G, Assiri A, Marzuuk N, Daef E, Abdelwahab S, Ahmed A, Mohamad I, Al-Eyadhy A, Alhaboob A et al (2016) Incidence and characteristics of hepatitis E virus infection in children in Assiut. Upper Egypt J Int Med Res 44(5):1115–1122
Zaki MES, Alsayed MAL, Abbas HRR, Ahmed DM, Ashry AYE (2020) Prevalence of hepatitis E virus in children with acute hepatitis: one Egyptian center study. Germs 10(2):88–94
Sayed IM, Abdelwahab SF (2022) Is hepatitis E virus a neglected or emerging pathogen in Egypt? Pathogens 11(11):1337
Stoszek SK, Abdel-Hamid M, Saleh DA, El Kafrawy S, Narooz S, Hawash Y, Shebl FM, El Daly M, Said A et al (2006) High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg 100(2):95–101
Blackard JT, Rouster SD, Nady S, Galal G, Marzuuk N, Rafaat MM, Daef E, El Din SS, Purcell RH et al (2009) Genotypic characterization of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol 46(2):140–144
Sayed I, El-Mokhtar M, Mahmoud M, Elkhawaga A, Gaber S, Seddek N, Abdel-Wahid L, Ashmawy A, Alkareemy E (2021) Clinical outcomes and prevalence of hepatitis E virus (HEV) among non-A-C hepatitis patients in Egypt. Infect Drug Resist 14:59–69
Boland F, Martinez A, Pomeroy L, apos, Flaherty N, (2019) Blood donor screening for hepatitis E virus in the European Union. Transfusion Medicine and Hemotherapy 46(2):95–103
Bi H, Yang R, Wu C, **a J (2020) Hepatitis E virus and blood transfusion safety. Epidemiol Infect 148:e158
Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P et al (2014) Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 384(9956):1766–1773
Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O’Riordan J, Boland F et al (2017) Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill 22(16):30514
Intharasongkroh D, Thongmee T, Sa-Nguanmoo P, Klinfueng S, Duang-In A, Wasitthankasem R, Theamboonlers A, Charoonruangrit U, Oota S et al (2019) Hepatitis E virus infection in Thai blood donors. Transfusion 59(3):1035–1043
Izopet J, Lhomme S, Chapuy-Regaud S, Mansuy JM, Kamar N, Abravanel F (2017) HEV and transfusion-recipient risk. Transfus Clin Biol 24(3):176–181
Favorov MO, Fields HA, Purdy MA, Yashina TL, Aleksandrov AG, Alter MJ, Yarasheva DM, Bradley DW, Margolis HS (1992) Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J Med Virol 36(4):246–250
Qashqari FS (2022) Seroprevalence of hepatitis E virus infection in Middle Eastern countries: a systematic review and meta-analysis. Medicina (Kaunas) 58(7):905
Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH et al (2006) Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg 100(2):89–94
Amer AF, Zaki SA, Nagati AM, Darwish MA (1996) Hepatitis E antibodies in Egyptian adolescent females: their prevalence and possible relevance. J Egypt Public Health Assoc 71(3–4):273–284
Mitsui T, Tsukamoto Y, Yamazaki C, Masuko K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H (2004) Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol 74(4):563–572
Goel A, Vijay HJ, Katiyar H, Aggarwal R (2020) Prevalence of hepatitis E viraemia among blood donors: a systematic review. Vox Sang 115(3):120–132
Ibrahim EH, Abdelwahab SF, Nady S, Hashem M, Galal G, Sobhy M, Saleh AS, Shata MT (2011) Prevalence of anti-HEV IgM among blood donors in Egypt. Egypt J Immunol 18(2):47–58
Hoad VC, Seed CR, Fryk JJ, Harley R, Flower RLP, Hogema BM, Kiely P, Faddy HM (2017) Hepatitis E virus RNA in Australian blood donors: prevalence and risk assessment. Vox Sang 112(7):614–621
Harvala H, Hewitt PE, Reynolds C, Pearson C, Haywood B, Tettmar KI, Ushiro-Lumb I, Brailsford SR, Tedder R et al (2019) Hepatitis E virus in blood donors in England, 2016 to 2017: from selective to universal screening. Euro Surveill 24(10):1800386
Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Hunter JG, Madden RG, Glasgow T et al (2014) Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther 40(11–12):1282–1291
Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S et al (2011) Hepatitis E virus and neurologic disorders. Emerg Infect Dis 17(2):173–179
Jha AK, Kumar G, Dayal VM, Ranjan A, Suchismita A (2021) Neurological manifestations of hepatitis E virus infection: an overview. World J Gastroenterol 27(18):2090–2104
Lhomme S, Abravanel F, Cintas P, Izopet J (2021) Hepatitis E virus infection: neurological manifestations and pathophysiology. Pathogens 10(12):1582
Hvas AM, Ostrowski SR, Frederiksen H, Kampmann P, Stensballe J (2021) SARS-CoV-2 vaccine-induced immune thrombosis and thrombocytopenia. Ugeskr Laeger 183(29):V01210002
Acknowledgements
None.
Funding
No governmental or society funding.
Author information
Authors and Affiliations
Contributions
Author (1) has proposed the hypothesis, the work out-frame and closely observed and revised the work. Author (2) provided scientific materials and primary revision of the work. Author (3) helped with the neurological assessment of the study population. Author (4) has provided the laboratory work-out for the study subjects. Author (5) contributed to the data collection, result analysis, manuscript writing, and submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research involved human subjects from whom informed consent was obtained prior participation in the study. Ethics Committee in Faculty of Medicine-Alexandria University approved the research with IRB NO 00007555-FWA NO: 00018699.
Consent for publication
All participants gave permission for being allocated as authors for publication.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanno, A.EF., Khouly, E.E., Abdel-Rauof, M. et al. Seroprevalence of hepatitis E viral infection among apparently healthy personnels and patients with certain neurological disorders in Alexandria University Hospitals. Egypt Liver Journal 13, 30 (2023). https://doi.org/10.1186/s43066-023-00266-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00266-8