Abstract
Background
Lymphoma is the most common primary hematological malignancy. FDG PET/CT has recently become the standard imaging modality for clinical management owing to its ability to provide precise, non-invasive anatomical and functional data. The purpose of this study was to highlight the role of 18F FDG-PET/CT in the management of lymphoma by monitoring treatment response, providing a guide for response-adapted therapy, and predicting the final therapeutic outcome.
Methods
This was a prospective monocentric cohort observational study in which thirty-three patients with histopathologically proved lymphoma of different types performed FDG-PET/CT scanning several times throughout the 24-month duration of the study. Early-stage interim SUVmax of the most active lesion (both nodal and/or extra-nodal) was measured and statistically analyzed together with data of the international prognostic index parameters and score.
Results
Among the included 33 patients of lymphoma, international prognostic index parameters and score together with the early-stage interim SUVmax of the predominant nodal and extra-nodal sites showed statistical significance in predicting the initial as well as the final treatment response after 24 months. Using ROC analysis, we could obtain cutoff values of SUVmax of the predominant nodal lesion of 2.75 (AUC 72%, 95% CI 0.42–1.0) and SUVmax of the predominant extra-nodal lesion of 3 (AUC 70.8% and 95% CI 0.23–1.0); therefore, SUVmax of higher than these values was related to stable or progressive disease, and lower levels than these values were related to complete or partial metabolic response based on Deauville 5-point scale and Lugano response criteria.
Conclusions
Early-stage interim PET-CT SUVmax of the predominant nodal and extra-nodal lesion could be a reliable parameter in predicting initial and final therapeutic outcome in lymphoma patients.
Similar content being viewed by others
Background
Lymphoma is the most common primary hematological cancer, with non-Hodgkin lymphoma accounting for 60% of all lymphomas and Hodgkin disease accounting for 40%. [1]. Clinical and radiological staging is essential for treatment planning and therapeutic outcome prediction [2].
18F-FDG positron emission tomography/computed tomography (18F-FDG PET/CT) has replaced anatomical imaging in managing FDG-avid lymphomas due to CT’s inability to differentiate between viable tumor and fibrosis in the post-therapy residual masses [3]. CT scanning provides morphology and size information but neglects cellular function, thus making it confusing to distinguish a reactive lymph node from a lymphoma-related one, especially at unreachable sites for biopsy and pathological evaluation [4].
Lymphoma tumor cells consume glucose, causing FDG phosphorylation and detection by PET scanners [5]. Combining a PET scanner with a multi-detector helical CT allows for more sensitive and specific imaging, accurately localizing FDG uptake and attenuation correction. [6]. PET measures FDG uptake using a standardized uptake value (SUV) providing functional information about tumor distribution and metabolic activity. SUVmax represents the highest tracer uptake in the voxel of interest [7].
The international prognostic index (IPI) and international prognostic score (IPS) are widely used to assess disease prognosis, aiding in the planning of therapeutic protocols, based on five parameters (age, initial disease stage, serum LDH, extra-nodal sites of involvement, and performance status) [8].
The Deauville 5-point scale (D5PS), an ordinal scale with 5 scores, rates FDG uptake at residual sites with the highest metabolic activity based on visual comparison with mediastinum and liver [9].
The Lugano classification is a standardized method for categorizing treatment responses in FDG-avid lymphoma into complete response, partial response, stable disease, or progressive disease [10].
Recent applications of PET/CT in lymphoma may be divided into pretreatment staging, restaging, therapy monitoring, post-therapy surveillance, and assessment of transformation [11].
Interim PET/CT is crucial for assessing treatment response between therapeutic cycles and, at the end, is often used for prognostication and early treatment escalation or de-escalation in response-adapted or risk-adapted treatment [12].
Few recent studies evaluate the diagnostic performance of lesion SUVmax of early-stage interim FDG-PET/CT in the management of lymphoma, with results still ambiguous and requiring further investigation [13, 14]. This study aimed at highlighting the role of PET/CT in the management of lymphoma and determining its value by monitoring the early response and predicting the final post-therapeutic outcome.
Methods
Patients
This prospective cohort observational study was conducted during the period from May 2021 to June 2023. Written consent was obtained from the included patients. The study was approved by IRB code MD.21.02.415. Thirty-three patients with histopathologically confirmed lymphoma of different types performed the first PET/CT (interim) scan after receiving the initial line of treatment according to each type of lymphoma (3–6 cycles of chemotherapy) and the follow-up (interim/end-of-treatment) PET/CT scan performed according to the standard treatment protocol to assess the therapeutic response.
Inclusion criteria were any age of both sexes suspected to have any type of lymphoma on a clinical and laboratory basis and on pathological evaluation using a bone marrow biopsy or biopsy from suspicious lesions. Demographic data are demonstrated in Table 1 including gender, age range, and risk factors.
Exclusion criteria were general contraindications for a CT scan, for example, an allergic reaction to contrast media, severe acute or chronic respiratory or circulatory failure, patients with hepatic or renal failure, and pregnant women. General contraindications for a PET scan are, for example, uncontrolled diabetic patients, patients with bony habitus above scanner dimensions, uncooperative and claustrophobic patients, recent chemotherapy in less than two weeks, surgery in less than 4 weeks, and radiotherapy in less than 3 months prior to scanning time.
Patient preparation
Full history, complete clinical examination, and laboratory tests are required to ensure serum creatinine and LDH levels. Patients were required to fast 6–8 hours while maintaining adequate hydration. The blood glucose level was measured to ensure it was ≤ 150–200 mg/dL. Renal function tests were required to avoid nephrotoxicity, serum creatinine < 1.5–2 mg/dL or glomerular filtration rate < 60 mL/min. The data of the IPI were collected and the IPS of each case was calculated. Patient body weight and height were measured using precise scales. A three-way valve system was used to inject the calculated proper dose of FDG depending on the patient’s weight (average adult dose: 0.7 mCi/kg) and then flush with 10 mL of saline. Scanning was done after about 60 min, and all patients were allowed to wait and stay in quiet and warm rooms and asked to void right before scanning.
Image acquisition
PET/CT was performed on an integrated scanner (G.E. Discovery ST 16 PET/CT scanner) that superimposes both CT and PET components in two sequential gantries, avoiding the need for patient motion for accurate fusion and co-registration of the CT and PET data. A low-dose non-enhanced CT scan was performed first for anatomical localization and attenuation correction. Then a whole-body PET study was performed within the same gantry, from the skull to the mid-thighs. The patient was in a supine position with arms extended and elevated above the head. The study takes about 30–45 min. Diagnostic enhanced whole-body CT scan was performed for better anatomical correlation and to obtain fused images. Using an automated injector for intravenous administration of low-osmolar iodinated contrast media (optray 350) 125 ml adult dose at a rate of 4 ml/sec, the study takes about 60–40 s. Scanning parameters were adjusted to obtain the required diagnostic information: tube current 110 mA, voltage 120 Kvp, slice thickness 3–5 mm, rotation time 2–3 min per bed position, pitch 1 and matrix 128 × 128 pixels. In 8 cases that require assessment of GIT lymphomatous infiltration, patients were given 200–800 mL of 2% oral contrast material divided at 45 and 15 min before the examination.
Imaging analysis and interpretation
All images were reviewed by two independent radiologists with ten- and three-year experience in PET/CT interpretation, respectively. Cross-linking of axial, coronal, and sagittal reformats for PET, CT, and combined images was performed . The SUV mean of the liver and mediastinal pool was measured. MIP images were checked for an overall initial scanning impression. Axial CT images were scrolled throughout, and any morphological abnormality detected was reported as regards its density and size which was then correlated with the corresponding fused PET/CT image. Abnormal tracer uptake was assessed visually and semi-quantitatively by putting region of interest (ROI) in the area of interest and measuring SUVmax. Anatomical abnormalities with an absent of tracer uptake were reported as well.
Nodal infiltration of all groups of lymph nodes throughout the entire body was carefully assessed, as well as the rest of the lymphatic system, including the spleen, tonsils, thymus, and bone marrow. Extra-nodal lymphomatous infiltration was also evaluated.
Grading of FDG uptake at the most metabolically active site is expressed using the Deauville 5-point scale which consists of Deauville Score 1: No uptake, Deauville Score 2: Uptake ≤ mediastinum, Deauville Score 3: Uptake > mediastinum but ≤ liver, Deauville Score 4: Moderately ↑ uptake > liver, and Deauville Score 5: Markedly ↑ uptake > liver [15].
Assessment of treatment response by Lugano response criteria is expressed as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The relationship between SUVmax of the most active lesion and treatment response vs international prognostic index factors, international prognostic score, and risk category was evaluated. The risk category is determined as follows: IPS0-1 → Low risk, IPS2 → Low-intermediate risk, IPS3 → High-intermediate risk, and IPS 4–5 → High risk [8].
Statistical analysis and data interpretation
Data were analyzed using the Statistical Package of Social Science (SPSS) program for Windows (Standard version 26). The normality of the data was first tested with a one-sample Kolmogorov–Smirnov test. Qualitative data were described using numbers and percent. Association between categorical variables was tested using Chi-square test. Fisher exact test and Monte Carlo test were used when expected cell count less than 5. Continuous variables were presented as mean ± SD (standard deviation) for normally distributed data and median (Min–Max) for non-normal data. The two groups were compared with independent t test (parametric) and Mann–Whitney test (nonparametric). For all above-mentioned statistical tests done, the threshold of significance is fixed at 5% level (p value). The results were considered significant when the p ≤ 0.05. The smaller the p value obtained, the more significant are. Interobserver agreement between the two radiologists was assessed using weighted kappa (κ) score. Kappa values were indicative of weak (0: 0.3), mild (0.3: 0.5), moderate (0.5: 0.7), and strong (0.7: 1) agreement.
The cutoff point of a test to discriminate responder cases from non-responder cases was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
This study included thirty-three patients (23 males and 10 females) with 43 years as mean age (range 22 -71) years. Thirteen patients (39.4%) were histopathologically diagnosed with HD, and twenty patients (60.6%) were diagnosed with NHL of which the most common subtype was DLBCL (16 patients). The most common initial disease stages by metastatic workup using contrast-enhanced pan CT and bone marrow biopsy were stages II and IV (Table 1).
We demonstrated that prior to receiving treatment, the highest percentage of patients (51.5%) belonged to the international prognostic risk category of low-risk group IPS 0–1, while only 6.1% of patients belonged to the high-risk group IPS 4–5. Those patients then received different lines of treatment according to therapeutic guidelines during the 24-month duration of our study. At the end of the study, based on Deauville score and Lugano response criteria, 22 (66.7%) patients developed a complete response (20 of them belong to the low-risk and low-intermediate risk groups), one patient (3%) developed a partial response, 3 (9.1%) developed stable disease, and 7 patients (21.2%) developed progressive disease (5 of them belong to high-intermediate and high-risk categories).
Our results showed a strong positive correlation between the initial treatment response and the international prognostic category and parameters. There is also a strong positive correlation between the initial treatment response and the first (interim) FDG-PET/CT SUVmax of the most active both nodal and extra-nodal sites (Table 2).
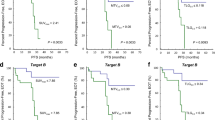
Our results revealed a negative correlation between the final therapeutic outcome after 24 months and some of the IPI parameters (age, initial disease stage, serum LDH, and extra-nodal sites of involvement). On the other hand, we found a strong positive correlation between the final therapeutic outcome vs performance status and the international prognostic risk category. There is also a strong positive correlation between the final therapeutic outcome and the first (interim) FDG-PET/CT SUVmax of the most active nodal and extra-nodal sites (Figs. 1 and 2). Hence, lower the SUVmax (nodal/extra-nodal), better the initial as well as final treatment outcomes and vice versa (Table 3). Interobserver analysis showed complete matching in the detection of nodal as well as extra-nodal sites. There was strong interobserver agreement of nodal and extra-nodal SUVmax measurement with kappa value κ 0.988 (95% CI 0.979–0.993), and κ 0.981 (95%CI 0.970–0.989), respectively (Table 4).
The ROC curve shows that the early-stage interim PET/CT SUVmax of the predominant nodal and extra-nodal sites are statistically significant discriminators of progressive/stable disease vs partial/complete response, with cutoff values of nodal SUVmax of 2.75 (AUC 72%, 95% CI 0.42–1.0) and extra-nodal SUVmax 3 (AUC 70.8% and 95% CI 0.23–1.0); therefore, SUVmax values higher than these values were related to stable or progressive disease, and lower levels than these values were related to complete or partial metabolic response based on Deauville-5-points scale and Lugano response criteria. Our metabolic values were adjusted to body weight (Table 5) and (Fig. 3). Demonstrative cases are shown in Figs. 4,5,6, and 7.
Male patient, 27 years old, diagnosed with NHL (DLBCL). His international prognostic risk category was low-intermediate risk. He received 6 cycles of chemotherapy. First (interim) PET/CT (hepatic reference 2.1) axial CT (A), axial MIP (B), axial fused PET/CT (C), sagittal CT (D), sagittal MIP (E) and sagittal fused PET/CT (F) images show metabolically active left inguinal LNs measuring (blue arrow) about 0.7 cm in short axis and achieving SUVmax 2.5 as well as multiple low metabolically active bone infiltrates (osteolytic and sclerotic) affecting multiple vertebral levels and sternum, the most active one (11th dorsal vertebral body) achieving SUVmax 2.8. The patient received 6 cycles of chemotherapy. Second (interim) PET/CT (hepatic reference 2.2) axial CT (A), axial MIP (B), axial fused PET/CT (C), sagittal CT (D), sagittal MIP (E) and sagittal fused PET/CT (F) images show complete metabolic resolution with of left inguinal LN (blue arrow) as well as the prior reported bony infiltrates. It was reported as complete response with Deauville score 2
Male patient, 39 years old, diagnosed with stage II NHL. His international risk category was low intermediate. He received first 4 cycles of chemotherapy. First (interim) PET/CT (hepatic reference 2.5); axial CT (A) & axial fused PET/CT (B) images show metabolically active right submandibular LN measuring about 1.5 cm in short axis and achieving SUVmax 4 (blue arrow). FNAC was done confirming the presence of malignant process. Patient resumed chemotherapy cycles and after 3 more cycles. Second (interim) PET/CT (hepatic reference 2.3); axial CT (A & C) and axial fused PET/CT (B & D) images show newly developed multiple bilateral upper deep cervical LNs, the most active left submandibular LN (yellow arrow) achieving SUVmax 12.3 and measuring about 1.2 cm in short axis. It was reported as progressive disease with Deauville score 5
Male patient, 34 years old, diagnosed with extra-nodal primary cecal lymphoma (NHL-DLBCL) by colonoscopic-guided biopsy. Metastatic workup was done confirming exclusive affection of caecum with no detectable nodal infiltration. His international risk category was low-intermediate risk. He received 6 cycles of chemotherapy. First (interim) PET/CT (hepatic reference 2); axial CT (A) & axial fused PET/CT (B) images show metabolically active enhancing ileo-cecal thickening measuring about 1.6 cm and achieving SUVmax 2.8. Colonoscopic biopsy was done confirming the malignant process. The patient received 4 cycles chemotherapy. Second PET/CT (hepatic reference 2.3); axial CT (A) and axial fused PET/CT (B) images show complete metabolic and morphologic resolution of the ileo-cecal thickening. It was reported as complete response with Deauville score 1
Male patient, 48 years old, diagnosed of HD (mixed cellularity) received different lines of chemotherapy then underwent autologous bone marrow transplant followed by radiotherapy, after 10 years; he developed weight loss and elevated serum LDH. His international prognostic risk category was high-intermediate risk. First post-transplant PET/CT (hepatic reference 1.7) axial CT (A & C) and axial fused PET/CT (B & D) images show metabolically active subcarinal LN (white arrows) measuring about 3.2 × 1.9 cm and achieving SUVmax 3 & metabolically active bilateral inguinal LNs (yellow arrows), the largest measuring about 9 mmm in short axis and achieving SUVmax 8.4. FNAC from right external iliac LNs was done confirming the relapse. The patient received 3 cycles chemotherapy. Second post-transplant PET/CT (hepatic reference 2.7) axial CT (A & C) and axial fused PET/CT (B & D) images show morphological and metabolic stationary course of the subcarinal (white arrows) and bilateral inguinal (yellow arrows) LNs. It was reported as stable disease with Deauville score 4
Discussion
Lymphoma represents around 4% of adult cancer cases and is the most prevalent primary hematological malignancy. 18F-FDG PET/CT allowed for the detection of both nodal and extra-nodal lymphomatous infiltration, allowing for accurate staging, treatment planning, response assessment, and therapeutic modification. Efforts to standardize PET acquisition and reporting have enabled it to become a gold state of the art in the management of malignant lymphoma providing accurate, non-invasive anatomical and functional data [16].
The strength of our study is that we investigated the predictive role of early-stage interim PET/CT as there were limited data in the previous publication, and we aimed at addressing the gap in the contradictory results. Our results revealed strong interobserver agreement in SUVmax measurement in both nodal and extra-nodal most active lesions. This highlights the promising role of PET/CT in the management of malignant lymphoma by monitoring the initial treatment response and providing predictors of the final therapeutic outcome.
Using the SUVmax of the predominant lesion, as it is the most accessible and reproducible parameter and is a marker of tracer concentration reflecting the tumor aggressiveness [17]. In our study, the ROC curve and statistical analysis revealed a strong positive correlation between early-stage interim SUVmax of the most active nodal/extra-nodal site and treatment response based on the Deauville score and Lugano response criteria. Our results revealed that the SUVmax value is directly correlated with tumor aggressiveness, as the higher the value, the more likely it is to develop a stable or progressive disease response, and the lower the value, the more likely it is to develop a partial or complete response. These findings were in agreement with Wang et al. [18] in a study that included 114 patients with histopathologically diagnosed lymphoma and stated that nodal SUVmax values were significantly different between treatment responder and non-responder groups. With the utility of ROC analysis, they found an area under the curve of 79%. This matches our results, in which we demonstrated ROC analysis with an area under the curve of 72%, but they did not provide a cutoff value.
Our findings align with those of a previous pilot study that compared the SUVmax of 122 high-grade lymphoma lesions, including nodal and extra-nodal, to international prognostic indices. The study found multiple positive correlative relationships, with a P value of 0.01. The high-risk group exhibited higher values of SUVmax, while intermediate- and lower-risk groups exhibited lower values [19].
Our results stated that there is a strong positive correlation between IPS and treatment response, as the majority of patients who developed a complete response belong to low-risk categories, while the majority of patients who developed progressive disease belong to high-risk categories. Our findings were concordant with a study conducted by Nyilas et al. [20], a 5-year retrospective cohort study that included 104 patients and studied the relations between the treatment response detected by interim PET/CT and IPS. It demonstrated that high IPS is strongly correlated with tumor progression and overall 2-year and 5-year survival decline during the follow-up.
A recent study that involved 42 patients with indolent lymphoma proposed a cutoff value that is very similar to what we observed during our research. AlShehry et al. [21] stated that SUVmax < 4.35 at interim PET/CT provided the best discrimination, with a progression-free survival (PFS) of 100% and a median survival time of 106.67 months compared with SUVmax ≥ 4.35 (P = 0.04), which had a PFS of 43.8% and a median survival time of 50.17 months. This cutoff was also appreciated in expecting overall survival at baseline, that is, 100% overall survival with baseline SUVmax < 4.35, versus 58.4% for SUVmax ≥ 4.35 (P = 0.13).
Our findings were contradictory to those of Alobthani et al., [13] who conducted a retrospective study enrolling 328 patients to evaluate the value of SUVmax in discriminating between aggressive and indolent types of NHL. They documented that the higher the SUVmax, the more suggestive of aggressive relapsing disease. They used ROC analysis and demonstrated SUVmax of 10 as the most balanced cutoff between aggressive and indolent NHL, with an area under the curve (AUC) of 86%, septicity of 94%, and sensitivity of 71%. This cutoff value discords with our study, in which we suggested a cutoff value of 2.75 for the predominant nodal lesion and 3 for the predominant extra-nodal lesion, which could be related to the small sample size and prospective nature of our research.
We investigated the predictive value of SUVmax for the most aggressive tumor in the early-stage PET/CT scan for both the initial and final treatment response. According to a retrospective 10-year study conducted by Texte et al. [14] involving 362 patients, early-stage scanning parameters yielded a more accurate prognosis prediction than follow-up/end-of-treatment scanning parameters. Additionally, they discovered that for recurrence prediction, SUVmax-based response assessment has a higher specificity than Deauville criteria.
Our study revealed a strong interobserver agreement for both nodal and extra-nodal SUVmax assessment, as well as complete matching in the detection of nodal and extra-nodal sites. These results were in agreement with a previous prospective cohort research consisting of sixty-four patients with high-grade B cell lymphoma, classical HL, or DLBCL that had been histologically established. Images were interpreted by two pairs of nuclear medicine physician and radiologists. Overall, the two reading teams’ interobserver agreement was strong. For all nodal sites combined, the kappa agreement was κ 0.94 (95% CI 0.88–1). For all extra-nodal sites combined, the kappa agreement was κ 0.91 (95% CI 0.82–0.99). For SUVmax, the 95% confidence intervals were 0.95 (0.91–0.97) [22].
The results of our interobserver agreement analysis aligned with those of a research that included 115 patients with DLBCL. Three experienced observers interpreted interim SUVmax, and the interobserver agreement was almost perfect. The kappa agreement κ ranged from 0.95 to 1 with a 3-year PFS of 82.28%. They reported that using interim SUVmax measurements showed higher prognostic accuracy and positive predictive value than the Deauville 5-point scale [23].
Our study has some limitations; firstly, it is a monocentric study with a small sample size. Secondly, the high cost of the scanning and inadequate health insurance compensations. Finally, there is limited availability of FDG isotope tracer.
Conclusions
Early-stage interim PET/CT could predict treatment response in lymphoma patients. SUVmax measurements of both nodal and extra-nodal lymphomatous infiltration are valuable clinical guides for tumor behavior before starting treatment plans and could help guide response-adapted therapy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG:
-
18F-Fluorodeoxyglucose
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- SUV:
-
Standardized uptake value
- ROC:
-
Receiver operating characteristic
- IPI:
-
International prognostic index
- IPS:
-
International prognostic score
- D5PS:
-
Deauville 5-point scale
- LDH:
-
Lactate dehydrogenase
- ROI:
-
Region of interest
- HD:
-
Hodgkin disease
- NHL:
-
Non-Hodgkin lymphoma
- DLBCL:
-
Diffuse large B cell lymphoma
- AUC:
-
Area under the ROC curve
- CI:
-
Confidence interval
- PFS:
-
Progression-free survival
References
Armitage JO, Gascoyne RD, Lunning MA, Cavalli F (2017) Non-hodgkin lymphoma. Lancet 390(10091):298–310
Bastos-Oreiro M, Muntanola A, Panizo C, Gonzalez-Barca E, de Villambrosia SG, Cordoba R, Lopez-Guillermo A (2020) RELINF: prospective epidemiological registry of lymphoid neoplasms in Spain. A project from the GELTAMO group. Ann Hematol 99:799–808
Juweid ME, Mueller M, Alhouri A, Arisheq MZ, Mottaghy FM (2021) Positron emission tomography/computed tomography in the management of Hodgkin and B-cell non-Hodgkin lymphoma: an update. Cancer 127(20):3727–3741
Cunningham J, Iyengar S, Sharma B (2017) Evolution of lymphoma staging and response evaluation: current limitations and future directions. Nat Rev Clin Oncol 14(10):631–645
Kilicoglu O, Sepay N, Ozgenc E, Gundogdu E, Kara U, Alomairy S, Al-Buriahi MS (2023) Evaluation of F-18 FDG radiopharmaceuticals through molecular docking and radiation effects. Appl Radiat Isot 191:110553
Shapira N, Scheuermann J, Perkins AE, Kim J, Liu LP, Karp JS, Noël PB (2021) Quantitative positron emission tomography imaging in the presence of iodinated contrast media using electron density quantifications from dual-energy computed tomography. Med Phys 48(1):273–286
Eskian M, Alavi A, Khorasanizadeh M, Viglianti BL, Jacobsson H, Barwick TD, Rezaei N (2019) Effect of blood glucose level on standardized uptake value (SUV) in 18 F-FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur J Nucl Med Mol Imaging 46:224–237
Cronin CG, Swords R, Truong MT, Viswanathan C, Rohren E, Giles FJ, Bruzzi JF (2010) Clinical utility of PET/CT in lymphoma. Am J Roentgenol 194(1):W91–W103
Al-Ibraheem A, Mottaghy FM, Juweid ME (2022) PET/CT in hodgkin lymphoma: an update. In: Seminars in Nuclear Medicine. WB Saunders
Ricard F, Cheson B, Barrington S, Trotman J, Schmid A, Brueggenwerth G, Korn R (2023) Application of the Lugano classification for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the ProLog consensus initiative (part 1—clinical). J Nucl Med 64(1):102–108
Ghesani N, Gavane S, HafezA, Kostakoglu L (2020) PET in Lymphoma. Clin Nuclear Med, 685–729
Minamimoto R, Fayad L, Advani R, Vose J, Macapinlac H, Meza J, Quon A (2016) Diffuse large B-cell lymphoma: prospective multicenter comparison of early interim FLT PET/CT versus FDG PET/CT with IHP, EORTC, Deauville, and PERCIST criteria for early therapeutic monitoring. Radiology 280(1):220
Alobthani G, Romanov V, Isohashi K, Matsunaga K, Watabe T, Kato H (2018) Value of f-FDG PET/CT in discrimination between indolent and aggressive non-hodgkin’s lymphoma: a study of 328 patients. Hell J Nucl Med 21(1):7–14
Texte E, Lequesne J, Tilly H, Jardin F, Vera P, Stamatoullas A, Becker S (2021) SUVmax-based assessment of PET response shows a superior specificity to Deauville criteria for predicting recurrence in Hodgkin’s lymphoma. Leuk Lymphoma 62(5):1088–1097
Hotta M, Minamimoto R, Gohda Y, Igari T, Yano H (2019) Impact of a modified peritoneal cancer index using FDG-PET/CT (PET-PCI) in predicting tumor grade and progression-free survival in patients with pseudomyxoma peritonei. Eur Radiol 29(10):5709–5716
Al Tabaa Y, Bailly C, Kanoun S (2021) FDG-PET/CT in lymphoma: where do we go now? Cancers 13(20):5222
Hirata K, Tamaki N (2021) Quantitative FDG PET assessment for oncology therapy. Cancers 13(4):869
Wang R, Zhu H, Chen Y, Li C, Li F, Shen Z, Xu B (2014) Standardized uptake value-based evaluation of lymphoma by FDG and FLT PET/CT. Hematol Oncol 32(3):126–132
Lim DH, Lee JH (2017) Relationship between dual time point FDG PET/CT and clinical prognostic indexes in patients with high grade lymphoma: a pilot study. Nucl Med Mol Imaging 51:323–330
Nyilas R, Farkas B, Bicsko RR, Magyari F, Pinczes LI, Illes A, Gergely L (2019) Interim PET/CT in diffuse large B-cell lymphoma may facilitate identification of good-prognosis patients among IPI-stratified patients. Int J Hematol 110:331–339
AlShehry NF, Shanker R, Zaidi SZA, AlGhmlas F, Motabi IH, Iqbal S, Ahmed M (2021) Role of 18F-fluorodeoxyglucose–positron emission tomography/computed tomography imaging in the prediction of prognosis in patients with indolent lymphoma: prospective study. JMIR FormRes 5(11):e24936
Husby T, Johansen H, Bogsrud T, Hustad KV, Evensen BV, Boellard R, Eikenes L (2022) A comparison of FDG PET/MR and PET/CT for staging, response assessment, and prognostic imaging biomarkers in lymphoma. Ann Hematol 101(5):1077–1088
Zhang Y, Fan Y, Ying Z, Song Y, Zhu J, Yang Z, Wang X (2018) Can the SUVmax-liver-based interpretation improve prognostic accuracy of interim and post-treatment 18F-FDG PET/CT in patients with diffuse large B-cell lymphoma? Leuk Lymphoma 59(3):660–669
Acknowledgements
Not applicable.
Funding
This study has no funding sources.
Author information
Authors and Affiliations
Contributions
SB participated in data collection, image interpretations, statistical analysis, writing and editing manuscript. GS participated in the design of study, data collection, image interpretation and statistical analysis. NB participated in statistical analysis and manuscript editing. AM participated in image interpretation and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Mansoura University’s Faculty of Medicine’s Ethical Committee gave its approval for the study and the patients’ participation. MD.21.02.415 is the code number.
Consent for publication
Informed consent for publication was obtained from all patients after assuring confidentiality.
Competing interests
Nothing to announce.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borg, S.M., Saleh, G.A., Batouty, N.M. et al. Role of interim positron emission tomography/computed tomography in assessment of lymphoma treatment response. Egypt J Radiol Nucl Med 55, 79 (2024). https://doi.org/10.1186/s43055-024-01247-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01247-6










