Abstract
Background
Studying cervical cancer is critical as it is the third most common gynecological malignancy. Therefore, a precise preoperative evaluation of the characteristics of the disease as well as prognosis may significantly aid in the diagnosis of cervical carcinoma as well as planning of its treatment.
The purpose of the study
To investigate if the value of apparent diffusion coefficient (ADC) could be interpreted as a prognostic indicator to predict cervical cancer aggressiveness prior to management.
Results
The value of ADC for high- and low-grade cervical cancer was statistically significantly different. Patients with histological grade I had significantly higher ADC in comparison with those with grade II (1.04 ± 0.07 vs. 0.82 ± 0.02 × 10−3 mm2/s; p < 0.001) and those with grade III (1.04 ± 0.07 vs. 0.67 ± 0.05 × 10−3 mm2/s; p < 0.001). In addition, patients with grade II had significantly higher ADC in comparison with those with grade III (0.82 ± 0.02 vs. 0.67 ± 0.05 × 10−3 mm2/s; p < 0.001).
Conclusions
Diffusion-weighted imaging (DWI) is one of the non-contrast imaging modalities which is identical for quantitative as well as morphological information. Combined DWI with apparent diffusion coefficient value can perform better in detecting cervical cancer and grading.
Similar content being viewed by others
Background
Cervical cancer is one of the major causes of cancer mortality among females globally, and many cases are linked to human papillomaviruses (HPV) infection, a prevalent sexually transmitted virus [1]. According to Global Cancer Statistics in 2018, cervical cancer is the fourth most pervasive cancer globally [2] and the second most prevalent in develo** countries in terms of occurrence as well as mortality, trailing only breast cancer [3].
Cervical cancer is usually diagnosed in females aging from 35 to 44, with the mean age during diagnosis being 50. It is not common to be developed in females under 20. In addition, > 20% of cervical cancer cases occur in females aging 65 and more [4]. The overall 5-year survival rate is 66%; however, cervical cancer is curable when diagnosed at an early stage, and as with most types of cancer, regular screening and early detection are keys to a good outcome [5]. Latest worldwide research has revealed substantial reductions in cervical cancer in high HPV vaccination rate cases [6].
Multiplanar conventional MRI is a fundamental problem-solving tool in evaluating variable female pelvic abnormalities when Transvaginal ultrasound is inconclusive [7]. Exciting advances have developed over the past several years in MR imaging technology [8]. The most worth mentioning is combined DWI with quantitative ADC measurement that can provide functional data in addition to anatomical details [9]. Comparatively to current MR Techniques that focus on the qualitative assessment of tissue characteristics, DWI permits noninvasive quantitative tissue assessment via the measurement of ADC values [10, 11]. Additionally, such non-enhanced mechanisms are beneficial for patients with impaired renal functions [12].
The mobility of water molecules in the extracellular space is connected to diffusion imaging, and ADC measures the degree of this diffusion [13]. As increased tissue cellularity limits water diffusion, so decreases ADC value. Generally, malignant tumors are characterized by more hyper-cellularity compared to benign lesions. Consequently, ADC values to aid in distinguishing between benign and malignant lesions [14]. High-grade cervical carcinoma has high cellularity and is anticipated to have diminished ADC values than the low-grade one. Thus, the expected clinical significance of ADC measurement in cervical cancer would be in the noninvasive prediction of tumor grading prior to deciding the treatment plan [13].
To our knowledge, there are scarce publications that investigated the diagnostic accuracy of ADC value in predicting cervical cancer grading; therefore, we conducted this study.
Methods
The present study is a cross-sectional analysis approved by the ethics committee in the interval from April 2019 and February 2022 in the two tertiary care hospitals (two university hospitals in upper Egypt and Delta i.e., multicenter study). Tumor grading was established by histopathology either after cervical biopsy or after radical hysterectomy (considered as the standard reference).
Patients
Thirty-three female cases were recruited from the Department of Obstetrics as well as the Department of Gynecology to the Department of Radiology for further MRI scanning with diffusion-weighted sequence to assess cancer grading according to ADC value.
Inclusion criteria
Cases that were pathologically diagnosed with cervical cancer.
Exclusion criteria
-
Contraindications for MRI include, for example, cases that have implanted magnetizable devices, claustrophobia, or pacemakers.
-
Cases with no pathology confirmation.
-
Proven benign cases.
MR imaging
MR examination using 1.5 T machines (Toshiba Vantage Titan, Japan) and (Ingenia; Philips) using pelvic-phased-array coils.
The protocol of MR imaging
MRI sequences
Localizer images in both axial and sagittal planes: 1. Sagittal T2WI. 2. Axial T2WI. 3. Coronal oblique T2WI 4. Axial T1WI. Axial T1-weight (TR/TE, 600/10 ms), axial T2-weight (TR/TE, 4000/105 ms), 4-mm slice thickness, gap 0.5 mm, and FOV 23 × 20 cm. Matrix 192 × 192, number of excitations [NEX] = 2, bandwidth = 89 kHz, flip angle = 90, number of averages = 1, coronal as well as sagittal T2-weighted, 5-mm slice thickness, gap 1 mm, and FOV 25 × 25 cm. Matrix 224 × 320, NEX = 2, bandwidth = 41.67 kHz, flip angle = 90, number of averages = 2.
Diffusion study
Axial DWI was conducted utilizing a single-shot echo-planar scanning via the application of three various b factors of 0, 500, as well as 1000 s/mm2. TR/TE = 6289/80 ms; 5-mm slice thickness and 1-mm interslice gap; matrix = 96 × 96, NEX = 8, bandwidth = 270 kHz, flip angle = 90, number of averages = 2.
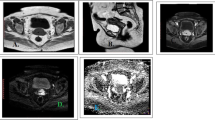
The images of DW images were utilized to calculate the values of ADC. Automatically, the measurements of ADC were quantified via establishing the region of interest (ROI) focusing on cervical carcinomas' solid component. The value of ADC was usually expressed in (× 10־3) square millimeters per second (Fig. 1).
A Sagittal T2 WI B axial T2 WI, A cervical mass is noted measuring 6.8 x 5.5 cm exhibiting iso to low signal intensity in T2, C It shows high signal on axial DWI D with low signal on the corresponding axial ADC maps (E). ADC value of the tumor was 1.01 x 10−3 mm2/s, pathology revealed highly differentiated squamous cell carcinoma, grade I
Imaging analysis
The analysis of images was conducted by two radiologists who have 15 and 7 year-experience, respectively, in the same major, and they collaboratively determined the final diagnosis, (both examiner radiologists were blinded to histopathological grading).
Quantitative as well as qualitative analyses of the masses examined were like the following:
Qualitative analysis
Restricted diffusions were identified through visualizing aberrant intermediate/bright signal intensity that is notable with higher values of b (0 → 500 → 1000) at "diffusion-weighted" (DW) images. In contrast, the ADC map demonstrated the lesion's diminished signal intensity (SI).
Quantitative analysis
Manually, the values of ADC were determined via ROI application at low SI regions on the ADC map, avoiding cystic/necrotic areas (Fig. 2).
A Sagittal T2 WI B axial T2 WI, A cervical mass is noted measuring 7.6 × 4 cm exhibiting iso to low intensity in T2, C It shows high signal on axial DWI D with low signal on the corresponding axial ADC maps (E). ADC value of the tumor was 0.67 × 10−3 mm2/s, histopathology revealed poorly differentiated squamous cell carcinoma, grade III
Statistical analysis
SPSS, version 20 was utilized to analyze data (SPSS Inc., Chicago, Ill, USA). Quantitative parametric data were expressed as standard deviations as well as means (+ -SD). The calculations of diagnostic tests like accuracy, NPV, specificity, PPV, and sensitivity were done utilizing version 18.10.2 of the Med Calc statistical software. In our investigation, pathological data was used as the gold standard. Considering the values of ADC, the ROC curve was utilized for predicting the malignant cervical lesions. The level of statistical significance was determined as follows: p-value ≤ 0.01 is considered highly significant, p-value ≤ 0.05 is significant, and p-value > 0.05 is non-significant (Fig. 3).
A Sagittal T2 WI B axial T2 WI, A cervical mass is noted measuring 7.2 × 4.6 cm exhibiting iso to high intensity in T2, C It shows intermediate signal on axial DWI D with low signal on the corresponding axial ADC maps (E) ADC value of the tumor was 0.84 × 10−3 mm.2/s, Histopathology revealed moderately differentiated squamous cell carcinoma, grade II
Results
The mean age of enrolled patients was 47.48 ± 9.90 years, with a range between 21 and 65 years. Out of the studied patients, 5 (15.2%) women were single, and 28 (84.8%) women were married (Table 1).
Histological grading was grade III (poorly or non-differentiated carcinoma), grade II (moderately differentiated), and grade I (well-differentiated) in 15 (45.5%), 4 (12.1%), and 14 (42.4%) patients, respectively (Table 2) (Fig. 4).
A comparative assessment of diffusion-weighted images as well as the average values of ADC with the grading of the pathological tumors was performed.
On DWI, all carcinomas examined demonstrated persistent intermediate to elevated signal with a diminished signal on the ADC map (restricted diffusion).
The mean values of ADC were 1.04 ± 0.07 × 10−3 mm2/s for grade I, 0.82 ± 0.02 × 10−3 mm2/s for grade II, as well as 0.67 ± 0.05 × 10−3 mm2/s for grade III cervical carcinoma (Table 3) (Fig. 5).
There were substantial differences between grades II from grade III (p < 0.001) and between grade I from grades (II and III) tumors (p < 0.001).
Additionally, analysis of data on the ROC curve demonstrated that ADC at cutoff point > 0.84 had 100% accuracy, 100% specificity as well as 100% sensitivity with the area under the curve was 1.00 for diagnosis of cervical cancer grade I. On the contrary, for diagnosis of cervical cancer grade II, ADC at cutoff point > 0.77 had 100% sensitivity, 52% specificity, and 57.8% accuracy, with the area under the curve being 0.617.
For diagnosis of cervical cancer grade III, ADC at cutoff point < 0.77 had 100% accuracy, 100% specificity, and 100% sensitivity, with the area under the curve being 1.00. (Tables 4, 5, and 6) (Fig. 6).
Discussion
When cervical cancer is detected and managed early, the prognosis is better. The majority of early-stage cervical malignancies have a better prognosis with elevated rates of survival. If cancer is discovered after it has progressed to other body regions (advanced stage malignancies), the prognosis is poor, and the probability of recurrence after therapy increases [15].
Accurate identification of cervical cancer grading, and staging allows for an appropriate treatment approach [16].
Conventional MR techniques cannot detect changes in cancer cell metabolism and physiology, which precede morphological changes. Functional MR imaging methods like DWI and ADC allow the assessment of structures functionally and more detailed information about the different physiological processes of the tumor microenvironment [11].
In our study, all cases of cervical cancer demonstrated restricted diffusion, with the average values of ADC being 0.85 ± 0.19 × 10−3 mm2/s, which is identical to the average ADC value revealed by other research; 0.82 ± 0.1 × 10−3 mm2/s in Mansour et al. [17] study, and 0.916 ± 0.15 × 10−3 mm2/s in Kuang et al. [18].
These results were also compatible with Hoogendam et al. [19] study, in which the average ADC of uterine cervical cancers in 20 cases was 0.86 ± 0.1 X 10−3 mm2/s, Nakmura et al. [20] found that the mean ADC value was 0.852 ± 0.1 × 10−3 mm2/s from a trial conducted on 80 patients already have cancer cervix and in a study carried out by Kuang F et al. [21], in which the average values of ADC for cervical cancer were 0.96 ± 0.15 × 10−3 mm2/s.
The range of ADC in our study was from 0.62 to 1.11 × 10−3 mm2/s, and thus it is not beyond the cutoff value between malignant as well as benign cervical lesions determined by previous research like Atstupėnaitė et al. [22], who set the 0.945 × 10−3 mm2/s ADC value as a cutoff for differentiating between malignant as well as benign cervical masses and Abo Gamra et al. [23] who found the 1.04 × 10−3 mm2/s ADC threshold was a cutoff value to differentiate between non-affected as well as cancer-affected cervical tissues.
We investigated in our current study the association between the mean value of ADC as well as the histopathological cervical cancer grading; we noticed a substantial inverse association between them, as higher-grade tumors demonstrated a decreased ADC value than lower grades (p = 0.001).
This finding is in harmony with prior research by Nakmura et al. (20), who illustrated that well-differentiated tumors are characterized by elevated ADC values in comparison with the non-differentiated tumors (p = 0.01).
We found that the mean value of ADC was significantly lower among patients with histological grade III, 0.67 ± 0.05 × 10−3 mm2/s, compared to those with grade II, 0.82 ± 0.02 × 10−3 mm2/s, and grade I was 1.04 ± 0.07 × 10−3 mm2/s.
Analysis of our data revealed that ADC value at cutoff point > 0.77 × 10−3 mm2/s had 100% sensitivity, 52% specificity, 22.3% PPV, as well as 100% NPV with an overall accuracy of 57.8% for the diagnosis of histological grade II from grades I and III. On the contrary, based on the value of ADC > 0.84 × 10−3 mm2/s as a cutoff point between grades III, II, and I, with an accuracy, specificity, sensitivity, NPV, as well as PPV of 100%, 100%, 100%, 100%, and 100%, respectively.
Conclusions
DWI is a non-contrast imaging modality similar to both quantitative and morphological information. Combined DWI with ADC value can perform better not only in detecting cervical cancer but also in grading. In our study, we could use the ADC value to discriminate between high-and low-grade tumors and thus an indicator of cervical cancer that may respond to chemotherapy. Further studies with a larger number of patients are recommended.
Availability of data and materials
The corresponding author is responsible for sending the used data and materials upon request.
Abbreviations
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
References
Mattiuzzi C, Lippi G (2020) Cancer statistics: a comparison between world health organization (WHO) and global burden of disease (GBD). Eur J Public Health 30(5):1026–1027
Zhang S, Xu H, Zhang L, Qiao Y (2020) Cervical cancer: epidemiology, risk factors and screening. China J Cancer Res 32(6):720
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Fontham ET, Wolf AM, Church TR, Etzioni R, Flowers CR, Herzig A, Guerra CE, Oeffinger KC, Shih YC, Walter LC, Kim JJ (2020) Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin 70(5):321–346
Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, Martinez Tyson D, Tortolero-Luna G, Fedewa SA, Jemal AM, Siegel RL (2021) Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin 71(6):466–487
Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, Martin D, Simms KT, Bénard É, Boily MC, Sy S (2020) Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet 395(10224):575–590
Dappa E, Elger T, Hasenburg A, Düber C, Battista MJ, Hötker AM (2017) The value of advanced MRI techniques in the assessment of cervical cancer: a review. Insights Imaging 8(5):471–481
Sala E, Rockall A, Rangarajan D, Kubik-Huch RA (2010) The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur J Radiol 76(3):367–385
Bollineni VR, Kramer G, Liu Y, Melidis C, DeSouza NM (2015) A literature review of the association between diffusion-weighted MRI derived apparent diffusion coefficient and tumour aggressiveness in pelvic cancer. Cancer Treat Rev 41(6):496–502
Rauch GM, Kaur H, Choi H, Ernst RD, Klopp AH, Boonsirikamchai P, Westin SN, Marcal LP (2014) Optimization of MR imaging for pretreatment evaluation of patients with endometrial and cervical cancer. Radiographics 34(4):1082–1098
Valentini AL, Miccò M, Gui B, Giuliani M, Rodolfino E, Telesca AM, Pasciuto T, Testa A, Gambacorta MA, Zannoni G, Rufini V (2018) The PRICE study: the role of conventional and diffusion-weighted magnetic resonance imaging in assessment of locally advanced cervical cancer patients administered by chemoradiation followed by radical surgery. Eur Radiol 28(6):2425–2435
Schieda N, Maralani PJ, Hurrell C, Tsampalieros AK, Hiremath S (2019) Updated clinical practice guideline on use of gadolinium-based contrast agents in kidney disease issued by the Canadian Association of Radiologists. Can Assoc Radiol J 70(3):226–232
Thoeny HC, Forstner R, De Keyzer F (2012) Genitourinary applications of diffusion-weighted MR imaging in the pelvis. Radiology 263(2):326–342
Shen SH, Chiou YY, Wang JH, Yen MS, Lee RC, Lai CR, Chang CY (2008) Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. Am J Roentgenol 190(2):481–488
Casali PG, Blay JY, Bertuzzi A, Bielack S, Bjerkehagen B, Bonvalot S, Boukovinas I, Bruzzi P, Dei Tos AP, Dileo P, Eriksson M (2014) Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl. 3):102–112
Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, Kehoe ST, Konishi I, Olawaiye AB, Prat J, Sankaranarayanan R (2019) Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet 145(1):129–135
Mansour SM, Raafat M (2017) Is there an added role for diffusion weighted imaging in the staging of cervical carcinoma? Egypt J Radiol Nucl Med 48(4):1131–1139
Kuang F, Yan Z, Li H, Feng H (2015) Diagnostic accuracy of diffusion-weighted MRI for differentiation of cervical cancer and benign cervical lesions at 3.0 T: comparison with routine MRI and dynamic contrast-enhanced MRI. J Magn Reson Imaging 42(4):1094–1099
Hoogendam JP, Klerkx WM, de Kort GA, Bipat S, Zweemer RP, Sie-Go DM, Verheijen RH, Mali WP, Veldhuis WB (2010) The influence of the b-value combination on apparent diffusion coefficient-based differentiation between malignant and benign tissue in cervical cancer. J Magn Reson Imaging 32(2):376–382
Nakamura K, Joja I, Nagasaka T, Fukushima C, Kusumoto T, Seki N, Hongo A, Kodama J, Hiramatsu Y (2012) The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol Oncol 127(3):478–483
Kuang F, Ren J, Zhong Q, Liyuan F, Huan Y, Chen Z (2013) The value of apparent diffusion coefficient in the assessment of cervical cancer. Eur Radiol 23(4):1050–1058
Atstupėnaitė V, Basevičius A, Krimelis A, Inčiūra A, Vaitkienė D (2011) Diffusion-weighted magnetic resonance imaging of cervical cancer. Acta medica Lituanica 18(4):139–146
Sinjawi HM, Gamra SH, Mutaleb MG (2018) Role of diffusion weighted MRI in diagnosis of cervical cancer. Egypt J Hosp Med 71(1):2411–2421
Acknowledgments
Not applicable.
Funding
No source of funding.
Author information
Authors and Affiliations
Contributions
SS, RH, AHM and SHF acted on the patient management, the study concepts and design as well as acquisition and analysis of images. SS, AHM, BMA and RH and contributed to preparation and interpretation of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants signed an informed consent after explaining to them the objective of the study. The study was approved by the Ethics Board of Aswan University. The ethics committee’s reference number is Ref. No.aswu /348/3/19.
Consent for publication
All patients included in this research were legible. They gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghardon, S.S.L., Hemida, R., Borg, M.A. et al. Correlative study between apparent diffusion coefficient value and grading of cervical cancer. Egypt J Radiol Nucl Med 53, 170 (2022). https://doi.org/10.1186/s43055-022-00850-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00850-9