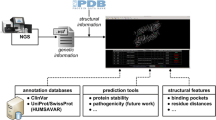
Abstract
Background
The huge number of detected somatic KIT mutations highlights the necessity of in silico analyses that are almost absent in the relevant medical literature. The aim of this study is to report the mutation spectrum analysis of exon 11 encoding the juxtamembrane (JM) domain of the KIT gene in a group of Syrian GIST patients.
Methods
Forty-eight formalin-fixed paraffin-embedded GIST tissue samples, collected between 2006 and 2016, were retrieved from the pathological archives and analyzed for KIT exon 11 mutations by DNA sequencing. Structural/functional impact of detected variants was predicted using several bioinformatic tools.
Results
Twenty-one different variants have been detected in intron 10, exon 11, and intron 11 of the KIT gene, eight of which were novel changes. Mutations in exon 11 of the KIT gene were detected in 28 of 48 (58.3%) GIST patients and predicted to be pathogenic and cancer promoting. Specifically, age above 60 was very significantly associated with the negative selection of deletion mutations (p = .007), a phenomenon that points to deletion severity.
Conclusions
Six bioinformatic tools have proved efficient in predicting the impact of detected KIT variations in view of published structural, experimental, and clinical findings.
Similar content being viewed by others
Background
Gastrointestinal stromal tumor (GIST) is the most common sarcoma in the GI tract and is distinguished from other mesenchymal tumors by CD117 (KIT) [1, 2]. This differential diagnostic immunohistochemical marker belongs to class III receptor tyrosine kinases (RTKs) characterized by extracellular, transmembrane, juxtamembrane, and bilobed kinase domains. The extracellular portion comprises five immunoglobulin-like domains involved in ligand binding and receptor dimerization, whereas the intracellular portion encompasses a ~ 30 amino-acid long regulatory juxtamembrane region and a kinase domain interposed by a ~ 80 amino-acid long kinase insert sequence [3, 4]. Ligand binding leads to receptor dimerization and tyrosine autophosphorylation in the juxtamembrane, kinase insert, and C-terminal tail, hence initiating signal transduction cascades and inducing cell growth and proliferation [3, 5].
CD117 is encoded by the KIT gene mapped to the chromosome region 4q12 and composed of 21 exons [6]. In GIST cases, KIT mutations most commonly cluster in exon 11 encoding the regulatory juxtamembrane (JM) domain of CD117 [3]. In the absence of ligand, wild-type JM domain inhibits KIT receptor dimerization and suppresses its kinase activity [3, 4]. Upon ligand binding, however, JM domain phosphorylation disrupts such autoinhibition and allows receptor activation [4]. Alternatively, mutations in exon 11 release JM suppression, induce ligand-independent constitutive receptor activation, and trigger uncontrolled cell division and carcinogenesis [3, 7].
The underlying biological changes in the receptor structure and function due to the KIT mutations as well as their impact on prognosis and therapeutic response have been enormously of interest to experimental and clinical studies [1, 8, 9]. Yet, the huge number of somatic KIT mutations that exceeded 2500 variants detected in more than 6000 GIST unique samples [6] precluded their individual analysis and highlighted the necessity of in silico analyses that are almost absent in the relevant medical literature. In this premier study within the Greater Middle East, we report the mutation spectrum of KIT exon 11, encoding the JM domain, in a group of Syrian GIST patients. Several bioinformatic tools have then been utilized to assess mutations pathogenicity in view of the structural, experimental, and clinical knowledge established in previous publications.
Methods
Specimens
All formalin-fixed paraffin-embedded GIST treatment-naïve tissue samples, collected from stomach, small or large intestine, or omentum and mesentery between 2006 and 2016, were retrieved from the pathological archives at Al Assad Hospital, Damascus University. Histopathological features such as cell type, mitotic rate, tumor grade, and immunohistochemical markers (CD117, CD34) were re-evaluated by two accredited pathologists. Areas containing more than 80% tumor cells were marked according to the hematoxylin–eosin (HE) staining and dissected by disposable sterile scalpels. The study was approved by the Research Ethics Committee of Damascus University, and written informed consent had already been obtained upon admission from all enrolled patients.
Direct sequencing
Genomic DNA was extracted using a Dual Genomic DNA Isolation Kit-Tissue (GeneDireX, Inc., Taiwan) according to the manufacturer’s instructions. Protocol modifications included using proteinase K (Thermo Fisher Scientific, USA) for tissue lysis and skip** the phase-separation step. DNA extracts quality was assessed using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., USA).
Exon 11 and ~ 60-bp flanking intronic regions of the KIT gene were amplified and sequenced using a forward primer 5′-CCA GAG TGC TCT AAT GAC TGA GA-3′ and a reverse primer 5′-AAA CAA AGG AAG CCA CTG GA-3′ (Eurofins Genomics, Germany) [10]. The final 50-μL PCRs included 0.2 μM of each primer, 25-μL HotStar PCR SuperMix (GeneDireX, Inc., Taiwan), and 5 μL of DNA extract. Thermal cycling was initiated at 94 °C for 2 min followed by 45 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 1 min; a final extension at 72 °C for 7 min was added. The 281-bp PCR amplicons were visualized on 2.5% agarose gel, purified using a High Pure PCR Product Purification Kit (Roche Diagnostics, Germany), and sequenced using a BigDye Terminator v3.1 Cycle Sequencing Kit on the ABI PRISM® 3100-Avant™ Genetic Analyzer (Applied Biosystems, USA).
Bioinformatic analysis
Exon and intron variants were identified, checked in the COSMIC [6], Ensembl [11], dbSNP [12], SNPedia [13], and LOVD [14] databases, and described according to the Human Genome Variation Society (HGVS) recommendations for Sequence Variant Nomenclature at the DNA and protein levels in relation to the NCBI Reference Sequence NG_007456.1 [15]. Structural/functional impact of all coding and noncoding variants were predicted using SIFT [16], PolyPhen-2 [17], fathmm [18], fathmm-MKL [19], AASsites [20], and MutationTaster [21] tools.
Statistical analysis
Statistical analysis was performed using web-based GraphPad QuickCalcs (https://www.graphpad.com/quickcalcs/; accessed Oct 2022). Associations between demographic, histopathological, and molecular variables were analyzed using Fisher’s exact test. P-value < 0.05 was considered statistically significant.
Results
Demographic and histopathological characteristics
Among 48 GIST patients included in our study, 29 (60.4%) patients were male, and 19 (39.6%) were female. The mean age at diagnosis was 52.6 years, ranging from 17 to 81 years. Most tumor cases originated from the stomach (27, 56.3%) followed by small intestine (15, 31.3%), omentum and mesentery (4, 8.3%), and large intestine (2, 4.2%). Microscopic evaluation revealed that most tumors had spindle cell type (27, 56.3%) followed by epithelioid type (12, 25%) and mixed spindle and epithelioid type (9, 18.8%). Mitotic rate was low (≤ 5/50 HPFs) in 8 (16.7%) cases, moderate (6–10/50 HPFs) in 25 (52.1%) cases, and high (> 10/50 HPFs) in 15 (31.3%) cases. Tumor grade was high in 40 (83.3%) cases and low in 8 (16.7%) cases. Among 48 CD117-positive tumors, 5 (10.14%) were focal, and 43 (89.6%) were diffuse, while CD34 was positive in 30 (62.5%) cases (Table 1).
Molecular variations
Mutations in exon 11 of the KIT gene were detected in 28 of 48 (58.3%) GIST patients, four of whom (14.3%) had additional variations in intron 11 and/or intron 10 (Table 1). Exon 11 mutations included one deletion in 10 (35.7%) patients, one deletion and one substitution in 11 (39.3%) patients, and one or two substitutions in 7 (25%) patients. No duplications or insertions were encountered. Introns 10 and 11 variations included one deletion in 1 of 4 patients and one or three substitution(s) in 3 of 4 patients (Table 1).
Overall, twenty-one different variants have been detected in intron 10, exon 11, and intron 11 of the KIT gene, eight of which were novel changes. Exon variations, mostly affecting codons 553–559, included nine single-nucleotide/amino acid substitutions (Table 2) as well as seven 1-bp, 6-bp, 12-bp, 21-bp, or 57-bp deletions leading to amino acid deletions, indels, or frameshift changes (Table 3). Intron single-nucleotide variations included four substitutions and one deletion (Table 4).
Bioinformatic analysis
Most substitutions detected in the JM domain of the KIT gene in our GIST patients were predicted to have deleterious (SIFT; 0) and probably/possibly damaging (PolyPhen-2; 0.627–1) effect on protein structure and function and to be cancer promoting (fathmm; < − 6.00) and disease causing (MutationTaster; probability 0.999; Table 2). However, in silico predictions obtained for p.(Val555Ile) and p.(Arg586Lys) substitutions were discordant. In addition, all deletions detected in the JM domain of the KIT gene in our GIST patients were predicted to be disease causing (MutationTaster; probability ≥ 0.9; Table 3). Among intronic variations, only c.1774 + 5G > A was shown to be pathogenic (fathmm-MKL; 0.98), disease causing (MutationTaster; probability 1), and likely changing the splicing pattern (AASsites; Table 4).
Statistical analysis
High tumor grade was significantly associated with age above 60 (p = 0.04) but independent of gender (p = 1.0), number of mutations (p = 1.0), and occurrence of deletions (p = 0.29) or any mutation (p = 0.44) in the JM domain of the KIT gene. Albeit not associated with gender (p = 0.54), the mutant status of the JM domain (p = 0.36), or number of JM domain mutations (p = 0.46), age above 60 was significantly associated with the partial absence of deletion mutations in favor of substitution mutations (p = 0.007). Contrariwise, gender, anatomic site, mitotic rate, and cell morphology were independent (p > 0.05) of the number of mutations and the occurrence of deletions or any mutation in the JM domain of the KIT gene.
Discussion
Due to its essential role of suppressing KIT proto-oncoprotein, the juxtamembrane (JM) domain releases its brake, when mutated, leading to proliferation promotion and apoptosis inhibition [3, 7]. This involvement of the JM domain-encoding exon 11 in GIST development is highly revealed by an average 86% mutation rate among KIT-mutated GIST patients [22,23,24,25]. In this study, exon 11 was mutated in about 58% of GIST patients approximating the average worldwide rate (60%) that spreads over the range 22–90% in more than a hundred studies. In discord with inherited variations, these reported frequencies of exon 11 somatic mutations among GIST patients were not correlated with geographic region according to our statistical estimation (data not shown). Nucleotide deletions and substitutions were the only mutation types detected in our GIST patients (Table 1); this accords with the majority of publications where deletions and substitutions are considerably reported contrary to insertions and duplications [26, 27]. In discord with numerous studies [2, 28], however, the combination of deletion-substitution mutation type was highly detected in our study group (Table 1).
Our bioinformatic analysis by SIFT, PolyPhen-2, fathmm, and MutationTaster tools revealed that all seven deletions and nine amino acid substitutions encountered in the JM domain were pathogenic (Tables 2 and 3). Despite their sole discordant predictions between the four tools, we suppose that Val555Ile and Arg586Lys mutations are more likely pathogenic according to the pathogenicity weight, conservation index, and large diverse databases adopted by relevant tools. Hence, the occurrence of any of these sixteen mutations in the KIT proto-oncogene might promote tumorigenesis. Their pathogenicity is further emphasized as high tumor grade seemed independent of type and number of mutations encountered in the JM domain of the KIT gene in our GIST patients (p > 0.05).
This virtual outcome is underlined by X-ray crystallographic reports that demonstrated the key role of the JM domain in general and its critical codons Tyr553, Trp557, Val559, Val560, and from Tyr547 to Gly565 in particular [29]. Notably, these aforementioned codons were affected by most presumably pathogenic mutations detected in our GIST patients, possibly disrupting JM function in stabilizing autoinhibited KIT and leading to cancer development [30, 31]. Moreover, the severity of these gain-of-function mutations in the JM domain, whether being substitutions or deletions, has already been manifested by in vitro and in vivo experimental evidence [30, 31]. Besides folding autonomously, the JM domain has been found to be highly conserved in class III receptor tyrosine kinases (RTKs) family. In fact, mutant JM domain demonstrated disordered secondary structure, reduced binding affinity to the kinase domain, less effective inhibition of receptor catalytic activity, and extremely rapid kinetics of ligand-independent receptor activation. Ultimately, the existence of substitution or deletion mutations in the JM domain of the KIT protein seemed sufficient for cell transformation [31].
Obviously, deletion mutations were specifically severe and seem to lower survival rate; this is supported by the following:
-
(i) Their high frequency in our cases with mutant JM domain (21 of 28; 75%).
-
(ii) Their apparent negative selection in favor of substitution mutations in our elderly GIST patients.
-
(iii) In addition to clinical evidence inferred by more than a dozen studies [8, 32, 33]
Interestingly, Trp557_Lys558del mutation was the most frequently encountered deletion (14 of 21; 67%). Furthermore, deletion mutations affected both codons 557 and 558 in 90% (19 of 21) of our cases. These observations point to the phenomenal severity of deletions in these amino acid positions.
GIST patients with mutated JM domain have significantly higher response rates to imatinib therapy [1, 9, 34, 35]; this was evident in seven of our patients including four responders with exon 11 mutations and three nonresponders with wild-type exon 11. Nevertheless, therapy information for the remaining GIST patients was not retrievable; hence, the association between imatinib response and the JM domain mutations could not be analyzed.
Our data indicated an association between high tumor grade and age above 60 (p < 0.05), an observation likely attributed to the accumulation of additional mutations over a longer lifespan in other loci of the genome. This explains the absence of association between tumor grade and the occurrence of JM domain mutations in our GIST patients (p > 0.05). Obviously, gender might not contribute to the tumor pathogenicity as it appeared independent of the occurrence, type, and number of JM domain mutations as well as tumor grade and age of onset. Nevertheless, it has been previously observed that men are slightly more likely to develop the disease and to have a worse prognosis than women [36, 37].
Extending the catalogue of KIT mutations in GIST, eight novel additional variations have been detected in our patients including two in intron 10, three in exon 11, and three in intron 11. Intriguingly, the two novel substitutions in Tyr553 and Lys581 occurred in one case and were deemed pathogenic by all utilized bioinformatic tools. This consists with the significance of Tyr553 amino acid in inhibiting KIT by interacting with Asp810 and Glu640 of kinase domain [29]. The third novel virtually pathogenic single-nucleotide deletion in exon 11 shifted the reading frame and incorporated a premature stop codon perhaps leading to the production of a truncated protein. The novel intron variations were supposedly neutral via in silico analysis except the one encountered twice and located within a splicing motif. These findings might not be conclusive as enrolling healthy individuals was beyond the scope of our study. Further biological and clinical studies might reveal the impact of these novel variations. Otherwise, genome-wide association studies in the future might classify such presumably neutral variants as polymorphisms.
Conclusions
In this study, we reported the mutation spectrum analysis of KIT exon 11, encoding the JM domain, in a group of Syrian GIST patients, where three supposedly pathogenic novel mutations were uncovered. Six bioinformatic tools have proved efficient in predicting the impact of detected KIT variations in view of published structural, experimental, and clinical findings. Utilizing computational analysis as a start point to guide research studies seems helpful to save human, financial, and material resources.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GIST:
-
Gastrointestinal stromal tumor
- HE:
-
Hematoxylin-eosin
- HGVS:
-
Human Genome Variation Society
- HPF:
-
High-power fields
- JM:
-
Juxtamembrane
- RTK:
-
Receptor tyrosine kinase
References
Dudzisz-Śledź M, Klimczak A, Bylina E, et al. Treatment of gastrointestinal stromal tumors (GISTs): a focus on younger patients. Cancers (Basel). 2022;14(12):2831.
Zheng S, Huang KE, Tao DY, et al. Gene mutations and prognostic factors analysis in extragastrointestinal stromal tumor of a Chinese three-center study. J Gastrointest Surg. 2011;15(4):675–81.
Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–49.
Roskoski R Jr. Structure and regulation of Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338(3):1307–15.
Mol CD, Lim KB, Sridhar V, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278(34):31461–4.
Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945–50.
Ma YY, Yu S, He XJ, et al. Involvement of c-KIT mutation in the development of gastrointestinal stromal tumors through proliferation promotion and apoptosis inhibition. Onco Targets Ther. 2014;7:637–43.
Haller F, Löbke C, Ruschhaupt M, et al. Increased KIT signalling with up-regulation of cyclin D correlates to accelerated proliferation and shorter disease-free survival in gastrointestinal stromal tumours (GISTs) with KIT exon 11 deletions. J Pathol. 2008;216(2):225–35.
Chen P, Zong L, Zhao W, et al. Efficacy evaluation of imatinib treatment in patients with gastrointestinal stromal tumors: a meta-analysis. World J Gastroenterol. 2010;16(33):4227–32.
Calibasi G, Baskin Y, Alyuruk H, et al. Molecular analysis of the KIT gene in gastrointestinal stromal tumors with novel mutations. Appl Immunohistochem Mol Morphol. 2014;22(1):37–45.
Zerbino DR, Achuthan P, Akanni W, et al. Ensembl 2018. Nucleic Acids Res. 2018;46(D1):D754–61.
Smigielski EM, Sirotkin K, Ward M, et al. dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res. 2000;28(1):352–5.
Cariaso M and Lennon G. SNPedia: a wiki supporting personal genome annotation, interpretation and analysis. Nucleic Acids Res. 2012;40(Database issue):D1308–12.
Fokkema IF, Taschner PE, Schaafsma GC, et al. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32(5):557–63.
den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564–9.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81.
Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9.
Shihab HA, Gough J, Cooper DN, et al. Predicting the functional consequences of cancer-associated amino acid substitutions. Bioinformatics. 2013;29(12):1504–10.
Shihab HA, Rogers MF, Gough J, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015;31(10):1536–43.
Faber K, Glatting KH, Mueller PJ, et al. Genome-wide prediction of splice-modifying SNPs in human genes using a new analysis pipeline called AASsites. BMC Bioinformatics. 2011;12(Suppl 4):S2.
Schwarz JM, Cooper DN, Schuelke M, et al. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–2.
Kang HJ, Ryu MH, Kim KM, et al. Imatinib efficacy by tumor genotype in Korean patients with advanced gastrointestinal stromal tumors (GIST): the Korean GIST Study Group (KGSG) study. Acta Oncol. 2012;51(4):528–36.
Chen J, Gundara JS, Haddad R, et al. Clinicopathological and molecular aspects of foregut gastrointestinal stromal tumours. ANZ J Surg. 2014;84(1–2):52–8.
Reichardt P, Demetri GD, Gelderblom H, et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer. 2016;16:22.
O’Brien KM, Orlow I, Antonescu CR, et al. Gastrointestinal stromal tumors, somatic mutations and candidate genetic risk variants. PLoS ONE. 2013;8(4): e62119.
Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563–70.
Xu Z, Huo X, Tang C, et al. Frequent KIT mutations in human gastrointestinal stromal tumors. Sci Rep. 2014;4:5907.
Rubió-Casadevall J, Borràs JL, Carmona-García MC, et al. Correlation between mutational status and survival and second cancer risk assessment in patients with gastrointestinal stromal tumors: a population-based study. World J Surg Oncol. 2015;13:47.
Mol CD, Dougan DR, Schneider TR, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279(30):31655–63.
Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80.
Chan PM, Ilangumaran S, La Rose J, et al. Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region. Mol Cell Biol. 2003;23(9):3067–78.
Capelli L, Petracci E, Quagliuolo V, et al. Gastric GISTs: analysis of c-Kit, PDGFRA and BRAF mutations in relation to prognosis and clinical pathological characteristics of patients - a GIRCG study. Eur J Surg Oncol. 2016;42(8):1206–14.
Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol. 2005;23(25):6190–8.
Gao J, Dang Y, Sun N, et al. C-KIT mutations were closely associated with the response to imatinib in Chinese advanced gastrointestinal stromal tumor patients. Med Oncol. 2012;29(5):3039–45.
Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016;52:173–80.
Rong J, Chen S, Song C, et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: a propensity score matching analysis. Biol Sex Differ. 2020;11(1):43.
IJzerman NS, van Werkhoven E, Mohammadi M, et al. Sex differences in patients with gastrointestinal stromal tumours: do they exist and does it affect survival? ESMO Open. 2022;7(6):100649.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The work presented here was carried out in collaboration between all authors. NPh and FM designed the research study. NPh and WH carried out the laboratory experiments. NPh, WH, and FM analyzed the data and interpreted the results. NPh wrote the manuscript. WH and FM revised the manuscript. All authors have contributed to, seen, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of Damascus University (Reference no. 3883, Date: 14 Sep 2015). Written informed consent was obtained from all enrolled patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pharaon, N., Habbal, W. & Monem, F. Bioinformatic analysis of KIT juxtamembrane domain mutations in Syrian GIST patients: jigsaw puzzle completed. J Egypt Natl Canc Inst 35, 25 (2023). https://doi.org/10.1186/s43046-023-00185-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00185-0