Abstract
Background
Acute myeloid leukemia (AML) is a cancer of hematopoietic progenitors characterized by gene mutations. The most popular deregulations are mutation and altered expression in the p53 gene, which is considered the guardian of the genome. Its activity is controlled by regulatory genes, e.g., alternate open reading frame (ARF), whose defects could affect p53 activity.
Aim
To study the effect of altered expression of p53 and ARF genes in de novo AML patients and correlate the results to the patients’ characteristics and outcomes.
Methods
Expression levels of p53 and ARF were assessed in 96 AML adult patients compared to 20 healthy controls using quantitative reverse-transcription PCR (RT-qPCR).
Results
There was significant up-regulation of p53 [77.6 (3.8–9528.3)] compared to controls [1.031 (0.210–9.051)], p < 0.001]. The expression level of ARF was significantly upregulated [6.2 (0.5–964.0)] compared to controls [0.854 (0.357–2.519), p < 0.001]. All of the low ARF expressers had low p53 overexpression, 61.1% of patients with high ARF expression had high p53 over-expression, and 38.9% with high ARF expression had low p53 over-expression (p < 0.001). ARF expression shows a trend of association with FLT3 mutation, as 89.3% with FLT3 mutation have high ARF expression (p = 0.080). Low p53 over-expression was seen in 77% of APL patients, while high p53 expression was associated with non-APL (p = 0.040). The median DFS of mutant NPM1 patients was higher than wild NPM1 (46.15 vs. 5.89 days, p = 0.045). Patients aged ≤ 50 years had better OS and DFS than those > 50 (p = 0.05, p = 0.035, respectively).There were no significant statistical associations between DFS and p53, ARF, and FLT3 mutations.
Conclusion
The p53 and ARF genes are overexpressed in de novo AML patients and they are interrelated. low p53 overexpression is associated with APL phenotype and t(15;17) and patients with t(15;17) had slightly better survival than patients with negative t(15;17) (p = 0.061). AML patients with mutated NPM1 had better DFS than wild NPM1 (p = 0.045). p53 pathway regulation can occur by many alternative ways rather than gene mutation.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease influencing hematopoietic progenitors leading to uncontrolled cell proliferation and loss of function. Gene mutations and deregulation of micro RNAs are among the characteristics of this disease [1, 2]. AML is the most common type of leukemia in adults, reaching about 80%. The prognosis of AML in adults is usually depressive despite increased curability up to 15% and 40% in patients above and below the age of 60, respectively [3,4,5].
. One of the most important gene mutations that directly impact AML pathophysiology and disease progression is the mutation of the TP53 gene located at chromosome 17p13, which acts as the guardian of genomic stability upon different cellular stresses [6,7,8]. As a genome guardian, p53 responds to oncogenic stresses by many mechanisms like cell cycle arrest and apoptosis to allow repair or eradication of the damaged cells [9,10,11].
P53 inactivation is an important requirement for the excessive growth of tumor cells [12]. Mutant p53 occurs in only 11.1% of the hematological malignancies [8] and 16% of de novo AML [13]. The activity of p53 is highly regulated either by mutational status, post-translational modifications, or interaction with different cofactors [14, 15].
Abramowitz et al. [16] Mouse double minute 2 (MDM2) and alternate open reading frame (ARF) are considered the most important p53 regulators. MDM2 inhibits p53 by promoting ubiquitination and proteasome-mediated degradation of p53, while ARF activates p53 by physically interacting with MDM2 to block its access to p53 [17].
Next-generation sequencing (NGS) is the most reliable and sensitive method to detect TP53 gene mutation [18, 19]. However, due to the economic burden in many countries, a lot of studies investigated other alternatives. Many studies involving bone marrow biopsy (BMB) samples and IHC reported an association between p53 protein overexpression and TP53 alterations. Several studies have discussed p53 overexpression in de novo AML, therapy-related AML, and MDS patients [20].
In this study, we focused on studying the effect of p53 and ARF genes expression using real-time PCR technique in de novo AML patients, which is a cost-effective, sensitive, and reliable method of gene overexpression. We also correlated the results with NPM1 gene mutation, patients’ characteristics, and outcomes.
Methods
Study population
This prospective study included 96 adult patients diagnosed with de novo AML admitted to the medical inwards at National Cancer Institute, Cairo University, from August 2016 to December 2021. The patients’ cohort comprised 55 males and 41 females aged between 18 and 77. Twenty healthy control subjects with comparable age and sex were enrolled. This study was approved by the ethical research committee of the Institutional Review Board, National Cancer Institute, Cairo University (Approval No. CP2301-503–008). N.B. Institutional Review Board number is updated according to the National Cancer Institute publication regulations.
The diagnosis of AML was based on morphologic assessment, cytochemical evaluation, immunophenoty** (IPT), and cytogenetics according to the French-American-British and World Health Organization criteria [21]. The median follow-up period was 1.81 months (range: 0.03–56.15). All patients were subjected to detailed history taking and complete clinical examination. Laboratory investigations included a complete blood picture using XT-1800i (Sysmex, BM-Egypt), morphological examination of the bone marrow aspirate (BMA) using Leishman stain and cytochemical stains (MPO/SBB, Dual esterase, and acid phosphatase) to identify AML subtypes. Immunophenoty** of blast cells in BMA samples was done using Navios Beckman Coulter, 6 color flow cytometry to confirm the diagnosis of AML with antibody panel of myeloid markers (MPO, CD13, CD33, CD117, and CD15), lymphoid markers (CD10, CD19 for B lymphoid series; CD3, CD2, CD4, CD8, CD7 and CD5 for T lymphoid series) and stem cell marker CD34 as well as HLA-DR on a routine basis. Subclassification of AML was done by a secondary antibody panel of markers; CD4, CD14, CD64, 11c, CD41, CD61, and Glycophorin A. Conventional karyoty**, Fluorescence In Situ Hybridization (when indicated) and conventional PCR for common genetic abnormalities t(8;21), t (15;17), inv (16), NPM1 mutation and FLT3 mutation. Successful karyotype analysis was done in 71/96 patients (74%). The patients were classified into specific cytogenetic risk groups by adding the results of recurrent genetic translocations performed by FISH analysis, e.g., t8;21 (8/96, 8.3%), inv16 (7/96, 7.3%), PML/RARA fusions (14/96, 14.6%), certain molecular techniques, e.g., FLT3 ITD (27/92, 29.3%), FLT3 TKD (20/92, 21.7%) and NPM1 mutations (34/93, 36.6%). Patients with low, intermediate, and high cytogenetic risk constituted 43.8%, 26%, and 30.2%, respectively. AML with recurrent genetic abnormalities represented 56.3%, and AML NOS represented 43.8%.
The patients were treated according to our institution’s regulations regarding induction and consolidation in AML adult protocol. The response to treatment was assessed clinically and by BM examination on days 14 and 28. The response was categorized as CR, partial response (PR), or refractory to treatment. Complete remission was defined following the standard criteria by Döhner et al. [22]. Disease-free survival (DFS) was measured from the date of CR to the date of relapse or death from any cause.
Detection of the expression of P53 and ARF genes
Total Cellular RNA extraction from Bone Marrow
After obtaining the patient’s consent, total RNA was extracted from the bone marrow samples of patients and controls using QIAamp RNA blood Mini Kit (Qiagen) according to the manufacturer’s instructions. Quantitation and purity assessment of RNA samples were done using the Nano Drop® (ND)-1000 spectrophotometer (Nano Drop Technologies, Inc. Wilmington, USA).
Real-time quantitative PCR (RT-qPCR) of P53 and ARF
Conversion of RNA to cDNA was done using the QuantiTect Reverse Transcription Kit (QIAGEN). A 10 μl of 2 × RT master mix was pipetted into each tube. The volume of the RNA sample was adjusted according to the RNA concentration in the sample to make the concentration of cDNA 50 ng/ μl. It was then completed by nuclease-free H2O until the total volume per reaction reached 20 μl. Thermal cycling conditions for optimization of reverse transcription kits were as follows: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 s [23, 24].
Quantitative reverse-transcription PCR (RT-qPCR) carried out using fluorescent TaqMan Gene Expression Assays (p53: Hs01034249_m1; ARF (GGA3): Hs01597822_m1; β-Actin as a reference gene, Thermo Fisher Scientific). Real-time PCR amplification was performed using the computerized thermocyclers (ABI step one Applied Biosystems). Relative quantification (RQ) of p53 and ARF genes was analyzed by the relative quantification Ct method (fold change) (2 − ΔΔCt) [25].
Statistical methods
Statistical analysis was done using IBM SPSS® Statistics version 23 (IBM® Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Pearson’s Chi-square test or Fisher’s exact test was used to examine the relation between qualitative variables. For not normally distributed quantitative data, comparison between two groups was made using Mann–Whitney test (non-parametric t-test). The spearman-rho method was used to test the correlation between numerical variables. Survival analysis was done using the Kaplan–Meier method, and comparison between two survival curves was made using log-rank test. The Receiver Operating Characteristic (ROC) curve was used for the prediction of cut-off values of the markers. Kappa test was used to evaluate the agreement between two markers. The biomarkers were assessed by calculating sensitivity, specificity, positive predictive values (PPV), negative predictive value (NPV), and accuracy. All tests were two-tailed. A p value < 0.05 was considered significant.
Results
Patients’ characteristics
Male patients represented 55 (57.3%), while females were 41 (42.7%), and their mean age was 41.0 ± 16.5 years. Our study included also 20 healthy non-patients controls, 12 males and 8 females with mean age 40.2 years and median 37.5 (29–55 years). Patients who suffered from fever, anemic manifestations, and gum hyperplasia were 55 (57.3%), 61 (63.5%), and (4.1%), respectively. Hepatomegaly was found in 13 patients (13.5%), while 15 patients (15.6%) had splenomegaly, and 19 (19.8%) had lymphadenopathy.
The median TLC was 17.74 (0.11–358.59) × 103/ mm3, the median HB concentration was 7.9 (3.5–14) gm/dL and the median platelets count was 34.5 (5–290) × 103/mm3. Also, the median of Peripheral blood blasts were 45.5 (1–98) % blasts.
Bone marrow aspirate showed hypercellular marrow in 78 patients (81.3%), and the median bone marrow blast percentage was 72.5 (20–97) %. FAB classification showed M0 class in 2 patients (2.1%), M1 in 15 (15.6%), M2 in 33 (34.3%), M3 in 14 (14.6%), M4 and M5 in 30 (30.3%), and M7 in 2 (2.1%). The myeloid phenotype was found in 64 patients (66.7%), and 30 patients (31.5%) showed a monocytic phenotype, while the megakaryocytic phenotype was found in only two patients (2.1%).
FISH analysis and routine molecular tests revealed that t(8;21) was positive in only 8/96 (8.3%), while t(15;17) was positive in 14 (14.6%), and 7 patients (7.6%) had inv 16. NPM1 gene mutation was positive in 34 patients (36.6%). FLT3/TKD mutation was present in 20 patients (21.7%), while FLT3/ITD mutant forms were found in 27 (28.1%). A normal karyotype was detected in 32 patients (45.1%). The patients were classified into high, intermediate, and low genetic risk according to Döhner et al. [22] as follows 29 (30.2%), 25 (26%), and 42 (43.8%), respectively. According to WHO classification, 54 patients (56.3%) were classified as AML with recurrent cytogenetic abnormality, while 42 (43.7%) were grouped into AML NOS.
Follow-up of response to treatment and survival over nearly 57 months revealed that 55 patients (57.2%) died before day 28 of starting chemotherapy, 38/41 of the remaining patients achieved complete remission, and three were refractory.
Expression levels of P53 and ARF genes in BM
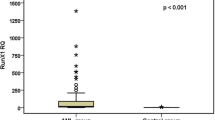
The expression level of p53 was significantly upregulated in all patients [median: 77.6, range: 3.8–9528.3] compared to controls [median: 1.031, range: 0.210–9.051, p < 0.001]. Also, the expression level of ARF gene was significantly upregulated [median: 6.2, range: 0.5–964.0] compared to controls [median: 0.854, range: 0.357–2.519, p < 0.001] (Table 1).
Diagnostic significance of p53 and ARF genes expression
The ROC curve analysis showed that the sensitivity, specificity, and AUC of p53 expression were 100%, 90%, and 0.997, respectively (p < 0.001) at a cut-off value of 3.018. The sensitivity, specificity, and AUC for the ARF gene were 81.3%, 85.7%, and 0.922, respectively (p < 0.001), at a cut-off value of 2.4.
The patients were classified into high and low p53 over-expressers according to the median of p53 fold change [47/95 (49.5%) patients vs. 48/95 (50.5%), respectively, p = 0.003]. Seventy-eight patients out of ninty six patients (81.2%) were considered ARF high expressers, and 18/96 (18.8%) were low expressers according to the cut-off of ARF gene expression (Fig. 1, Table 2).
There was a significant association between p53 and ARF gene expression, as 47/77 (61.1%) patients with high ARF expression showed high P53 over-expression, while 30/77 (38.9%) showed low p53 over-expression. All of the low ARF expressers showed low p53 over-expression (p < 0.001, Table 3).
Association between p53 expressions and patients’ characteristics
p53 low over-expression was found in 17/33 patients (51.5%) with mutant NPM1 and 31/59 (52.5%) with wild NPM1 with no significant difference (p = 0.925). In patients with high p53 over-expression, FLT3 and NPM1 mutations were detected in 47.8% and 48.5%, respectively. Nearly 77% of patients with t(15;17) had low p53 over-expression, while high p53 expression was seen in patients negative for t(15;17) (p = 0.040). On cytogenetic analysis, 53.1% of cytogenetically normal patients had high over-expression of p53, while 52.6% of patients with abnormal karyotype had low p53 over-expression (p = 0.631). Low p53 over-expression was more frequent in patients with recurrent genetic abnormalities compared to AML NOS patients who had high p53 over-expression (p = 0.183). The relations between p53 gene expression and different genetic mutations are shown in Table 4
Association between ARF gene expression and patients’ characteristics
Most patients had high ARF expression (75/93, 80.6%), mutant NPM1 patients 28/34 (82.4%) and wild NPM1 47/59 (79.7%) were both associated with high ARF gene expression, but the difference was insignificant (p = 0.752). There was a trend to significant relation between total leucocytic count and ARF expression. High ARF expression was found in 41/56 patients (73.2%) with TLC < 30,000/mm3, 24/26 (92.3%) in TLC 30–100,000/mm3, and 13/14 (92.9%) with TLC > 100,000/mm3 (p = 0.078). High ARF expression was apparently associated with lower hemoglobin concentration. Patients with Hb ≤ 10 gm/dL (75/90, 83.3%) had high ARF expression (p = 0.078). High ARF expression was seen in 40/78 patients (51.3%) with hypercellular marrow, while 10/18 patients (55.6%) and hypo- and normocellular BM had low ARF expression (p = 0.601).
ARF expression was associated with FLT3 mutation as 89.3% of patients with FLT3 mutation have high ARF expression with a trend toward statistical significance (p = 0.080). The relations between ARF with gene mutations are shown in Table 4.
High expression of ARF gene was noticed in patients with positive PML/RARA translocations (9/14, 64.3%), while negative patients were also nearly associated with high ARF expression (69/82, 84.1%), and the relation shows a statistical trend (p = 0.078). High ARF expression was found in 28/32 patients (87.5%) with CN-AML and 31/39 (79.5%) with an abnormal karyotype (p = 0.370). Also, high ARF expression was detected in 42/54 patients (77.8%) with recurrent cytogenetic abnormalities and 36/42 patients (85.7%) with AML NOS (p = 0.323).
There were no significant relations between p53 and ARF expression levels and age, sex, organomegaly, disease symptoms, other PB parameters, BMA cellularity and blasts, FAB classifications, and IPT markers (Table 4).
Relation between NPM1 and FLT3 and lymphadenopathy
Wild NPM1 genes were found to be associated more with wild FLT3 TKD (42/69, 60.9%), while mutant NPM1 was less with mutant FLT3 TKD 5/20 (25%); however, the relation was not significant (p = 0.246). Meanwhile, patients with wild NPM1 were associated with wild FLT3 ITD (40/62, 64.5%), while patients with mutant FLT3 ITD were associated with wild NPM1 (17/27, 63.0%, p = 0.888). Mutant NPM1 was more frequent in patients with lymphadenopathy (15/18, 83.3%) than those without lymphadenopathy who had more wild NPM1 (44/75, 58.7%, p = 0.051).
Association between p53 expression and response to treatment
p53 expression was not significantly associated with treatment response (p = 0.241), where 57.2% of patients with low p53 over-expression had CR at day 28. In comparison, 42.8% of patients who didn’t achieve CR had high p53 over-expression.
Association between ARF gene expression and response to treatment
There was no significant association between ARF gene expression and response to treatment (p = 0.316), where 76.3% of patients who achieved CR had a high expression compared to 81.3% of those who didn’t. For NPM1 mutant patients, 24/34 (70.6%) patients achieved CR versus 34/59 (57.6%) patients with wild NPM1 (p = 0.214).
Survival analysis
The median follow-up period was 1.81 months (range: 0.03–56.15). At the end of the study, 12 patients died, and the median overall survival was 1.71 months (0.03–3.39). The overall survival (OS) was significantly affected by age, FAB classification, and the detection of t(15;17). Patients 50 years or younger had better OS than those above 50 (3.13 vs. 1.12 months, respectively, p = 0.05). Patients with acute myeloid leukemia and monocytic leukemia (AML M4&M5) had better OS than other FAB subclasses M3, [M1/M2/M7] (3.59 vs. 1.7 and 1.0 months, respectively p = 0.088). Patients with t(15;17) had slightly better survival than patients with negative t(15;17) (p = 0.061). The hazard ratio analysis which describes the effect of gene mutations (P53, ARF and NPM) on OS and DFS is illustrated in Tables 5 and 6.
Disease-free survival (DFS) was affected by age; patients ≤ 50 years had better DFS than those > 50 years (23.32 vs. 2.3, respectively, p = 0.035). Also, patients with t(15;17) had better DFS than those negative for t(15;17) (p = 0.008). Patients with mutated NPM1 had better DFS than wild NPM1 (46.1 vs. 5.8 months, respectively, p = 0.045). Patients with low-risk cytogenetics had better DFS than intermediate and high-risk groups (46.1 vs. 2.6 and 8.7 months, respectively), and the difference shows a trend toward statistical significance (p = 0.08).
Multivariate Cox regression analysis which investigate the statistically significant associations between patients clinical data and DFS, leading to the most accurate cause of enhanced DFS in those patient cohort which showed that the only independent factor affecting DFS was the patients’ age ≤ & > 50 years (p = 0.036) with 95% CI (1.058—5.595) (Fig. 2).
Survival analysis in relation to P53 expression
The median OS of patients with high p53 over-expression vs. low over-expression was 1.91 and 1.71 months, respectively, and the difference was not statistically significant (p = 0.109). DFS of patients with p53 gene high and low expression was found to be statistically insignificant, as shown in Fig. 3.
Survival analysis in relation to ARF genes expression
The median OS of patients with high ARF expressers vs. low expressers was 1.71 vs. 1.41, respectively, p = 0.783). DFS of patients with ARF high and low expression was found to be statistically insignificant (Fig. 3).
Survival analysis in relation to NPM1 genes expression
The median OS of wild NPM1 patients was more than twice compared to those with mutant NPM1 (2.86 vs. 1.05 months); however, the difference was not statistically significant (p = 0.703). The median DFS was significantly better in patients with mutant NPM1 than those with wild NPM1 (46.15 vs. 5.89, p = 0.045).
Discussion
p53 gene mutations were only detected in 5–8% of de novo AML cases [26]. Hence, p53 pathway deregulations could be done in many alternative ways rather than mutations. Overexpression of the p53 gene in AML patients could be related to p53 mutation. Mutated p53 is largely associated with p53 overexpression, according to Kubbutat et al., who attributed high p53 expression in mutant p53 due to a deficiency of inducible proteins that degrade p53 protein like MDM2 and E3 ligase [27]. Upon exposure to cellular stress via oncogenes or DNA damage, prompt and rapid upregulation and activation of p53 occur via inhibition of post-translational modifications, which promote p53 overexpression to arrest the growth of cancerous blast cells [28].
According to Chen et al., ARF gene activation and overexpression could explain the rapid activation and overexpression of the p53 gene in response to cellular stress through ULF-mediated ubiquitylation, which establishes the interaction between p53 and MDM2 [29]. ARF gene activation and overexpression of p53 gene can also be slowly and irreversibly mediated by epigenetic modifications of the ARF gene locus [30, 31].
Abramowitz et al. [17] analysis revealed that p53 is not functional as an activating transcription factor in cytogenetically normal-AML as they did not find gene induction related to various p53 functions, including cell cycle arrest, apoptosis, DNA repair, and oxidative stress defense [16].
Like others [29, 32], we found that p53 and ARF expression and activation are cornerstone barriers against oncogenesis.
We found that p53 gene was overexpressed in de novo AML patients like in other reports, which assessed p53 overexpression by quantifying p53 protein in BMB samples with IHC technique and scoring system in de novo AML and therapy-related myeloid patients [20, 33]. Also, in agreement with Eischen et al., we found high p14 ARF tumor suppressor gene expression in many AML samples [34].
Like other reports [16, 35], we found that p53 and ARF gene overexpression were not correlated with the AML subtypes, cytogenetically-based prognosis, morphological stage, or by type of molecular mutation. We noticed in APL patients, low overexpression of p53 was significantly present. This finding was in agreement with Abramowitz et al., who explained that functional inactivation of p53 pathway was due to defective acetylation [16]. Also, it was found that p53 was overexpressed in more than 60% of CN-AML and APL patient samples, and it was expressed in approximately 75% of the leukemic cells in the BM sample [16].
NPM1 is a molecular chaperone linked to favorable prognosis in AML [36]. The incidence of NPM1 mutations in our study was similar to that reported by Assi et al., who also found that high p53 expression was associated with mutant NPM1 [20]. Like others [36, 37], we found that NPM1 mutations were associated with low genetic risk and longer DFS.
In accordance with Assi et al., we found that nearly half of the patients with p53 high expression were associated with mutated FLT3 and NPM1.
In earlier studies of AML and MDS, Loghavi et al., Saft et al., and McGraw et al. found p53 overexpression detected by IHC to be associated with inferior survival [38,39,40]. In contrast to others [16, 33], we could not find a possible association between patients’ survival, DFS with the expression of p53, and ARF in our patient cohort.
Conclusion
We can conclude that p53 and ARF genes are overexpressed in de novo AML patients and they are interrelated. low p53 overexpression is associated with APL phenotype and t(15;17) and patients with t(15;17) had slightly better survival than patients with negative t(15;17) (p = 0.061).
AML patients with mutated NPM1 had better DFS than wild NPM1(p = 0.045). So p53 pathway regulation can occur by many alternative ways rather than gene mutation. Further enhanced research on p53 pathway regulators should be done to conclude the effect of p53 and ARF overexpression in AML patients and their impact on patient outcomes.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AML:
-
Acute myeloid leukemia
- APL:
-
Acute promyelocytic leukemia
- ARF:
-
Alternative reading frame
- AUC:
-
Area under the curve
- BM:
-
Bone marrow
- BMB:
-
Bone marrow biopsy
- CBC:
-
Complete blood count
- CN:
-
Cytogenetically normal
- CR:
-
Complete remission
- DFS:
-
Disease-free survival
- FAB:
-
French-American-British
- FLT3:
-
Fms-like tyrosine kinase 3
- HB:
-
Hemoglobin
- HLA-DR:
-
Human leukocyte antigen –DR
- IHC:
-
Immunohistochemistry
- Inv16:
-
Inversion of chromosome 16
- IPT:
-
Immune-phenoty**
- ITD:
-
Internal tandum duplication
- MDM2:
-
Mouse double minute 2
- MDS:
-
Myelodysplastic syndrome
- MPO:
-
Myeloperoxidase
- ND:
-
Nano Drop
- NGS:
-
Next-generation sequencing
- NOS:
-
Not otherwise specified
- NPM1:
-
Nucleophosmin
- NPV:
-
Negative predictive value
- OS:
-
Overall survival
- PPV:
-
Positive predictive values
- PR:
-
Partial response
- RT-qPCR:
-
Quantitative reverse-transcription polymerase chain reaction
- SBB:
-
Sudan black B
- TKD:
-
Tyrosine kinase domain
- TLC:
-
Total leucocytic count
- ROC:
-
Receiver Operating Characteristic
References
Chen J, Odenike O, Rowley JD (2010) Leukaemogenesis: more than mutant genes. Nat Rev Cancer 10(1):23–36
Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447
Bain BJ (2019) Béné MC morphological and immunophenotypic clues to the WHO categories of acute myeloid leukaemia. Acta Haematol 141(4):232–244
Naymagon L, Marcellino B, Mascarenhas J (2019) Eosinophilia in acute myeloid leukemia: overlooked and underexamined. Blood Rev 36:23–31
Medeiros BC, Chan SM, Daver NG, Jonas BA, Pollyea DA (2019) Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol 94(7):803–811
Chipuk JE, Green DR (2006) Dissecting p53-dependent apoptosis. Cell Death Differ 13:994–1002
Lozano G (2016) The enigma of p53. Cold Spring Harb Symp Quant Biol 81:37–40
Petitjean A, Achatz MI, Borresen-Dale AL et al (2007) TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 26:2157–2165
Vogelstein B, Sur S, Prives C (2010) p53: the most frequently altered gene in human cancers. Nat Educ 3(9):6
Dolgin E (2017) The most popular genes in the human genome. Nature 551(7681):427–431
Kastenhuber ER, Lowe SW (2017) Putting P53 in context. Cell 170(6):1062–1078
Hahn WC, Weinberg RA (2002) Rules for making human tumor cells. N Engl J Med 347(20):1593–1603
Robles AI, Jen J, Harris CC (2016) Clinical outcomes of TP53 mutations in cancers. Cold Spring Harb Perspect Med 6(9):a026294
Hafner A, Bulyk ML, Jambhekar A, Lahav G (2019) The multiple mechanisms that regulate P53 activity and cell fate. Nat Rev Mol Cell Biol 20(4):199–210
Farkas M, Hashimoto H, Bi Y, Davuluri RV, Resnick-Silverman L, Manfredi JJ et al (2021) Distinct mechanisms control genome recognition by p53 at its target genes linked to different cell fates. Nat Commun 12(1):484
Abramowitz J, Neuman T, Perlman R, Ben-Yehuda D (2017) Gene and protein analysis reveals that p53 pathway is functionally inactivated in cytogenetically normal Acute Myeloid Leukemia and Acute Promyelocytic Leukemia. BMC Med Genomics 10:18
Kung CP, Weber JD (2022) It’s getting complicated—a fresh look at p53-MDM2-ARF triangle in tumorigenesis and cancer therapy. Front Cell Dev Biol 10:818744
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374(23):2209–2221. https://doi.org/10.1056/NEJMoa1516192
Ross L, Peter JM (2019) Valk next-generation sequencing in the diagnosis and minimal residual disease assessment of acute myeloid leukemia. Haematologica 104(868):71. https://doi.org/10.3324/haematol.2018.205955
Assi R, Gur HR, Loghavi S, Konoplev SN, Konopleva M, Dever N et al (2018) P53 protein overexpression in de novo acute myeloid leukemia patients with normal diploid karyotype correlates with FLT3 internal tandem duplication and worse relapse-free survival. Am J Hematol 93(11):1376–1383. https://doi.org/10.1002/ajh.25255
Daniel AA, Attilio O, Robert H et al (2016) Revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al (2010) diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474
Sabry D, Mostafa A, Mekawey D, Altaib Z, Shamaa A, Hany A, Abd-El-Hassib DM, Ibrahim NE, Khalifa WA (2018) An experimental model: intrauterine adhesion versus subendometrial fibrosis. Biomed Res 29(17):3311–3318
Sanders R, Mason DJ, Foy CA, Huggett JF (2013) Evaluation of digital PCR for absolute RNA quantification. PLoS ONE 8(9):e75296. https://doi.org/10.1371/journal.pone.0075296
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Ley TJ, Miller C, Ding L et al (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368:2059–2074
Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387:299–303
Brooks CL, Gu W (2006) p53 ubiquitination: Mdm2 and beyond. Mol Cell 21:307–315
Chen D, Shan J, Zhu W, Qin J, Gu W (2010) Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nat Vol 464:624–627
Kruse JP, Gu W (2009) Modes of p53 regulation. Cell 137:609–622
Matheu A, Maraver A, Serrano M (2008) The Arf/p53 pathway in cancer and aging. Cancer Res 68:6031–6034
Zindy F et al (1998) Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev 12:2424–2433
Cleven J, Nardi V, Young Ok C, Goswami M, Cin P, Zheng Z, Iafrate A et al (2015) High p53 protein expression in therapy-related myeloid neoplasms is associated with adverse karyotype and poor outcome. Mod Pathol 28:552–563
Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL (1999) Disruption of the ARF–Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 13:2658–2669
Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC et al (2010) Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol 28(15):2529–2537
Falini B, Mecucci C, Tiacci E et al (2005) Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 352:254–266
Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, Linch DC (2008) The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Medical Research Council Adult Leukaemia Working Party. Blood 111(5):2776–2784
Loghavi S, Al-Ibraheemi A, Zuo Z et al (2015) TP53 overexpression is an independent adverse prognostic factor in de novo myelodysplastic syndromes with fibrosis. Br J Haematol 171:91–99
Saft L, Karimi M, Ghaderi M et al (2014) p53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q). Haematologica 99:1041–1049
McGraw KL, Nguyen J, Komrokji RS et al (2016) Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse clinical outcome in myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica 101:e320–e323
Acknowledgements
Not applicable.
Funding
The research is self-funded.
Author information
Authors and Affiliations
Contributions
RN designed and performed the experiments. NM and HA contributed to the interpretation of the result and the analysis of the results. RN, HA, SS to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All eligible individuals agreed to voluntary participation and signed an informed consent form.
Consent for publication
All authors read and approved the final manuscript and all persons gave their informed consent.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nabil, R., Elshazly, S.S., Hassan, N.M. et al. The expression level of ARF and p53 in AML patients, and their relation to patients' outcome. Egypt J Med Hum Genet 24, 26 (2023). https://doi.org/10.1186/s43042-023-00410-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00410-4