Abstract
Tobacco mosaic virus (TMV) is widely recognized as one of the most important plant viruses, causing significant agricultural losses in terms of both quality and yield worldwide each year. This study demonstrated the biosynthesis of copper oxide nanoparticles (CuONPs) using orange peel extract for effective control of TMV infection both in vitro and in vivo. After treatment with CuONPs (100 mg/L) for 2 h, TMV particles exhibited evident fragmentation in vitro, reducing infectivity on tobacco plants. Similarly, the application of CuONPs on Nicotiana benthamiana (N. benthamiana) positively impeded viral replication and accumulation in vivo. Interestingly, the expression of systemic resistance-related genes (PR1, PR2, ERF1, and JAZ3) in the host plant was up-regulated by CuONPs treatment, supporting that CuONPs activated plant immunity to inhibit TMV. Importantly, the application of CuONPs (100 mg/L) did not exhibit any toxic effects on tobacco and, instead, resulted in the promotion of chlorophyll content, as well as an increase in the fresh weight and dry weight of the plant when compared to the control treatment. Overall, we proposed that the appropriate concentration of CuONPs (100 mg/L) can directly break viral particles by passivating, boost plant immunity by stimulating systemic acquired resistance (SAR), and provide nutritional supplements to promote plant growth.
Similar content being viewed by others
Background
Plant viruses have infected more than 700 crops, resulting in declining global crop yields (Chen and Wei 2020). It has been estimated that plant viral infections incur annual losses exceeding $60 billion to the global agricultural sector (Bos 2000). Tobacco Mosaic virus (TMV), a model plant virus, holds significant importance among plant viruses (Scholthof et al. 2011). It exhibits a broad host range, infecting over 125 plant species across nine different families (Yang and Klessig 1996; Islam et al. 2018), especially Solanaceae crops (Cai et al. 2020). Currently, multiple methods of controlling viral disease have been developed, including biological control, chemical control, and disease-resistant varieties breeding (Cai et al. 2019). However, to date, no effective method has been developed to control this plant disease (Chen et al. 2012). Therefore, exploring innovative technologies that can effectively manage viral diseases is imperative to enhance crop production and quality.
The term “nanoparticles (NPs)” refers to materials with diameters in the range of 100 nm, which possess exceptional properties at the nanoscale (Rajwade et al. 2020). Due to their special physical and chemical properties, NPs are gradually applied in many fields, such as medical treatment, electronics, cosmetics, food, energy, and agriculture. Simultaneously, there has been a growing interest in inorganic nanoparticles (INPs), particularly those incorporating bio-essential metals such as Cu, Mg, and Zn (Kanakari and Dendrinou-Samara 2023). The antiviral activity of INPs primarily manifests through the inhibition of viral entry into plant cells or the suppression of viral replication post-entry (Farooq et al. 2021). In the field of agriculture, numerous studies have demonstrated the antiviral activity of nanomaterials against plant viruses. For instance, El-Dougdoug reported that pre-treatment with Silver nanoparticles (AgNPs) three or seven days prior to inoculation with Potato Virus Y (PVY) or Tomato Mosaic Virus (ToMV) significantly reduced virus concentration and infection rate (El-Dougdoug et al. 2018). In addition, Fe2O3NPs and TiO2NPs sprayed on the leaf surface have strong antiviral properties and can effectively prevent the infection of Turnip mosaic virus (TuMV) into the new leaves (Hao et al. 2018). Foliar application of nano-CuO, MnO, and ZnO can also reduce fusarium wilt and verticillium wilt on tomatoes without causing physiological toxicity to them (Elmer and White 2016). In a word, more and more applications of NPs contribute to controlling plant disease and enhancing food production (Vrandecic et al. 2020). All these researches also have proved that NPs have broad prospects in agriculture (Duhan et al. 2017).
In recent years, the synthesis of nanoparticles often involved physical and chemical methods, which had some drawbacks, such as environmental pollution, toxicity concerns, limited productivity, and high costs (Chakraborty et al. 2022). However, a more recent focus on biosynthesis has emerged, which involves synthesizing non-toxic and stable compounds from natural or environmental resources (Sarkar et al. 2012; Jadhav et al. 2018; Jadoun et al. 2020). One promising approach that has gained attention is using plant extracts for biosynthesis, particularly citrus fruit extracts, due to their abundant phytochemical constituents (Tshireletso et al. 2021). This method offers several advantages, including simple operation, rapid speed, and large-scale production (Tshireletso et al. 2021). Moreover, numerous studies have documented the successful synthesis and application of various metal/metal oxide nanoparticles, including Cu, Au, Ag, CuO, ZnO, and Fe2O3, by using environmentally friendly synthesis techniques (Sarkar et al. 2012, 2014, 2017; Jadhav et al. 2018; Chakraborty et al. 2022).
In many metallic materials, copper-based compounds have been widely used to control plant pathogens (George 1935). Chen et al. showed that the toxicity of CuNPs (LD50 of 413 mg/kg) was lower than that of soluble Cu (LD50 of 110 mg/kg) (Chen et al. 2006). These findings highlight the feasibility and application potential of using biosynthesis CuONPs in plant protection. Here, we bio-synthesized CuONPs by orange peel. The effects of CuONPs at different concentrations on TMV infection were studied in vitro and in vivo. We proposed a synergistic effect of our CuONPs on the control of TMV disease, which is composed of passivation viral particles of TMV, stimulates plant immunity, and enhances tobacco growth with increasing chlorophyll contents. Herein, our study presents a novel viral control method by spraying biosynthesized CuONPs (100 mg/L) that promote plant growth but also investigates the mechanisms of CuONPs that inhibit TMV.
Results
Biosynthesis and characterization of CuONPs
CuONPs were biosynthesized by orange peel following a previous description (Tshireletso et al. 2021), which has also been shown in Fig. 1a. To enhance the understanding of the inhibitory effect of CuONPs on TMV, a series of essential characterizations had been performed. The biosynthesized CuONPs were characterized by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) to investigate their shape and size. Figure 1b, c showed the irregular spherical CuONPs successfully prepared from orange peel, with average sizes of about 50 nm. In this study, CuONPs were in a dispersed state with a weak agglomeration phenomenon (Okpara and Fayemi 2019). Next, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) were employed to analyze the elemental composition and surface groups of the as-prepared CuONPs in detail. As shown in Fig. 1d, the peaks at 3445/cm were attributed to the O-H stretching for alcoholic and phenolic functional groups. The peaks at 1626/cm corresponded to the C double bond O stretching of the carbonyl group. Interestingly, the infrared peaks at 533/cm corresponded to the formation of metal oxide (Cu-O) (Sisira et al. 2022). For the XPS spectra, there are three major peaks of C, O, and Cu, suggesting that CuONPs here mainly composed of C, O, and Cu. Since CuONPs were synthesized from the orange peel as a reducing agent, the product contained rich organic functional groups (Vinothkanna et al. 2022). Moreover, the phase structures of the as-prepared CuONPs samples were investigated by the X-ray diffraction (XRD) analysis. As shown in Fig. 1f, the diffraction peaks of CuONPs matched well with CuO (JCPDS: 48-1548) (Siddiqui et al. 2018). The characteristic diffraction peaks at 35.47° (002) in CuONPs represented CuO, indicating the formation of CuONPs (JCPDS: 48-1548).
The effect of CuONPs on TMV virus particles in vitro
The interactions between materials and plant viruses primarily encompass direct viral inactivation, impediment of viral entry into plant cells, and subsequent interference with viral replication. Considering that CuONPs might influence all stages of TMV infection, we firstly proposed to determine whether CuONPs have direct toxic interactions on TMV.
TMV particles were respectively treated with CuO (100 mg/L) and CuONPs (100 mg/L) for 2 h as described in the method. The changes of TMV particles were observed by TEM and the results were shown in Fig. 2a. There was no significant damage to TMV particles after deionized water treatment. For CuO (100 mg/L) treatment, TMV particles almost maintained their original forms without obvious damage except for some aggregation. For CuONPs (100 mg/L), TMV particles were fractured. Moreover, the damage of TMV morphology for CuONPs treatment was much more severe than CuO treatment (Fig. 2a). Then, the treated TMV virions were used to inoculate N. benthamiana. As shown in Fig. 2c–f, CuONPs-treated TMV virions have lower infection ability on N. benthamiana. Compared with water, both CuO and CuONPs exhibited significant inhibition on the initial infection of TMV. We also further compared the accumulation of TMV at 5 days post-inoculation (dpi) between different treatments. As shown in Fig. 2e, f, the relative expression of TMV demonstrated that CuONPs (100 mg/L) had the best inhibitory effect on TMV multiplication at 5 dpi. Furtherly, immunoblot against GFP for the total of TMV-GFP reconfirmed that CuONPs (100 mg/L) had the best inhibitory effect on TMV at 5 dpi.
Passivation effect of CuO/CuONPs on TMV. a TEM images of TMV particles treated with CuO/CuONPs in vitro. The red arrows indicate TMV damage. b Process by CuO/CuONPs inhibit TMV infection in tobacco plants. c, e GFP fluorescence in the newly emerged and inoculated leaves after CuO/CuONPs treatment of 2 and 5 dpi passivation. d Fluorescence intensity of the inoculated leaves after 2 dpi of passivation treatment. f Relative expression of TMV in new leaves after 5 dpi of passivation. g The total proteins of tobacco leaf at 5 dpi were extracted for each treatment and immunoblots against GFP were used to detect the content of TMV-GFP. The top half part is the immunoimprint, which is used to detect the GFP tag of TMV-GFP. The below half part is the Coomassie bright blue stain, which is used to detect the protein content. h Accumulation of TMV-related proteins in tobacco leaves. The CuO10, CuO100, CuONPs10, and CuONPs100, respectively, represent CuO (10 mg/L), CuO (100 mg/L), CuONPs (10 mg/L), and CuONPs (100 mg/L). Significant differences between the control and treatment groups are marked with ** P < 0.01, or * P < 0.05
The effect of CuONPs on TMV in vivo
Therapy effect of CuONPs on TMV
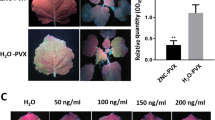
To accurately and further evaluate the antiviral activities of CuONPs, the therapeutic effects of CuO and CuONPs on tobacco mosaic disease were calculated as shown in Fig. 3a. The corresponding pictures of TMV infection were recorded after the material was sprayed (1 dpi and 4dpi) (Fig. 3b–d). After 1 dpi, all tobacco plants treated with antivirals showed inhibition on TMV compared with the control. Among them, CuONPs (100 mg/L) had the best effect (Fig. 3c). TMV proliferation in tobacco was observed at 4 dpi (Fig. 3d), and it was clear that the expression of TMV (Fig. 3e) in CuO (100 mg/L) and CuONPs (100 mg/L) were lower than others, indicating that CuONPs (100 mg/L) had therapeutic effects on infected plants.
Therapeutic effects of CuO/CuONPs on TMV. a Process by CuO/CuONPs inhibit TMV infection in tobacco plants. b, d GFP fluorescence in the newly emerged and inoculated leaves after CuO/CuONPs treatment of 1 and 4 dpi therapeutic. c Fluorescence intensity of the inoculated leaves after 1 dpi of therapeutic treatment. e Relative expression of TMV in new leaves after 4 dpi of therapeutic treatment. f The total proteins of tobacco leaf at 4 dpi were extracted for each treatment and immunoblots against GFP were used to detect the content of TMV-GFP. The top half part is the immunoimprint, which is used to detect the GFP tag of TMV-GFP. The below half part is the Coomassie bright blue stain, which is used to detect the protein content. g Accumulation of TMV-related proteins in tobacco leaves. The CuO10, CuO100, CuONPs10, and CuONPs100, respectively, represent CuO (10 mg/L), CuO (100 mg/L), CuONPs (10 mg/L), and CuONPs (100 mg/L). Significant differences between the control and treatment groups are marked with ** P < 0.01, or * P < 0.05
Protective effect of CuONPs on TMV
To evaluate the antiviral activities of CuONPs accurately and furtherly, the protective effects of CuONPs against TMV were also measured, as shown in Fig. 4a. After 2 dpi, all tobacco plants treated with antivirals can inhibit TMV infection, and CuONPs (100 mg/L) had most potent suppressive effect on TMV infection. The accumulation of TMV was also detected at 5 dpi (Fig. 4d, e). CuONPs (100 mg/L) had an inhibitory effect on TMV proliferation at 5 dpi, significantly better than CuO (10 mg/L and 100 mg/L). These results indicated that spraying CuONPs (100 mg/L) in advance had a certain protective effect on plants.
Protective effects of CuO/CuONPs on TMV. a Process by CuO/CuONPs inhibit TMV infection in tobacco plants. b, d GFP fluorescence in the newly emerged and inoculated leaves after CuO/CuONPs treatment of 2 and 5 dpi protective. c Fluorescence intensity of the 2 dpi of protective treatment. e Relative expression of TMV in new leaves after 5 dpi of protective treatment. f The total proteins of tobacco leaf at 5 dpi were extracted for each treatment, and immunoblots against GFP were used to detect the content of TMV-GFP. The top half part is the immunoimprint, which is used to detect the GFP tag of TMV-GFP. The below half part is the Coomassie bright blue stain, which is used to detect the protein content. g Accumulation of TMV-related proteins in tobacco leaves. The CuO10, CuO100, CuONPs10, and CuONPs100, respectively, represent CuO (10 mg/L), CuO (100 mg/L), CuONPs (10 mg/L), and CuONPs (100 mg/L). Significant differences between the control and treatment groups are marked with ** P < 0.01, or * P < 0.05
Expression of plant several defense marker genes
To further evaluate the influence of CuONPs on plant immunity, the expression levels of salicylic acid (SA) marker genes (PR1 and PR2), key genes involved in ethylene (ET) signal transduction (EIN3 and ERF1), jasmonic acid (JA) transduction pathway enzyme coding genes (JAZ3), and abscisic acid (ABA) phytohormone biosynthetic genes (NCED3) were detected after spraying water, CuO, and CuONPs 1 day. We found that the hormone signaling pathways of SA, ABA, and JA were significantly up-regulated by CuONPs. In detail, exposure to 100 mg/L CuONPs, the expression levels of the genes in tobacco leaves were increased 2.79 times (PR1), 2.39 times (PR2), 3.61 times (ERF1), and 1.34 times (JAZ3) compared with the control, respectively (Fig. 5a–c). There was no significant difference in the expression level of NCED3 after exposure to 100 mg/L CuONPs compared with the control (Fig. 5d). The results collectively suggested that the stimulation of CuONPs positively regulated plant immunity response through the activation of SA, ET, and JA related signaling pathways.
The expression of SA, ET, JA, and ABA signaling pathway-related genes induced by CuO/ CuONPs. Relative expression level of a PR1 and PR2, b EIN3 and ERF1, c JAZ3, and d NCED3 after biosynthesized CuO/CuONPs treatments. The CuO100 and CuONPs100 represent CuO (100 mg/L) and CuONPs (100 mg/L), respectively. Significant differences between the control and treatment groups are marked with ** P < 0.01, or * P < 0.05
Plant growth response
With the rapid development of nanotechnology, the toxicities of nanomaterials were also focused (Hoseinzadeh et al. 2017). Given that CuONPs could play as a viricide in this study, there was an urgent need to evaluate their potential adverse reactions and risks of use in plants, especially their effects on plant growth. Then, the plant height, fresh weight, dry weight, and chlorophyll content of N. benthamiana were calculated after spraying different concentrations of CuO and CuONPs, respectively.
Water-treated tobacco plants were used as blank controls. Plant physiological changes were recorded after 12 d of leaf exposure (Fig. 6). There was no noticeable difference among different treatments in plant height (Fig. 6a). Compared with the CK, the chlorophyll content of the CuO (10 mg/mL and 100 mg/mL) and CuONPs (10 mg/mL and 100 mg/mL) treatments all significantly increased about 30% (Fig. 6b). In the other hand, CuONPs (10 mg/L and 100 mg/L) could promoted the fresh weight of tobacco (Fig. 6c). CuONPs (100 mg/L) could significantly promoted the dry weight of tobacco (Fig. 6d). All these results indicated that CuONPs (10 mg/L and 100 mg/L) could increase the growth of N. benthamiana without obviously toxicity effects.
Exposure response of tobacco plants to CuO and CuONPs. Effects of CuO (10 mg/L and 100 mg/L) and CuONPs (10 mg/L and 100 mg/L) on a plant height, b chlorophyll content, c fresh weight, and d dry weight of tobacco. The CuO10, CuO100, CuONPs10, and CuONPs100, respectively, represent CuO (10 mg/L), CuO (100 mg/L), CuONPs (10 mg/L), and CuONPs (100 mg/L). Significant differences between the control and treatment groups are marked with ** P < 0.01, or * P < 0.05
Discussion
The present study demonstrated the potent antiviral activity of CuONPs against TMV in vitro and in vivo. CuONPs (100 mg/L) effectively disrupted the structure of TMV in vitro (Fig. 2), resulting in reduced infectivity. Within plant cells, the virus’s coat protein formed a complex with NPs, resulting in the inhibition of viral replication due to the strong electrostatic charge and high affinity of NPs towards the viral genome (Vargas-Hernandez et al. 2020). The possible explanation lay in TMV’s negatively charged nature (Cai et al. 2019), while CuONPs exhibited a high density of positive charges (Zhu et al. 2016). This reason could partially explain the therapeutic and protective effects of CuONPs. Interestingly, the therapeutic and protective effects of CuONPs (100 mg/L) against TMV disease were demonstrated in vivo (Figs. 3 and 4), showing that CuONPs (100 mg/L) have an effect on both diseased and non-diseased tobacco. The protective effect of CuONPs suggested that they possessed the ability to modulate plant immunity in response to viral infections. Correspondingly, we observed the up-regulations of plant defense marker genes, which were associated with SA, JA, and ET after CuONPs treatment (Fig. 5). The excitement of our study lay in the uniform enhancement of plant growth in N. benthamiana through the application of CuONPs at a concentration of 100 mg/L (Fig. 6). In totally, we proposed that the application of CuONPs can suppressed TMV infection without inducing discernible phytotoxic effects by directly disrupting the structure of TMV and eliciting plant immune responses through modulation of SA, JA, and ET signaling pathways.
Previous research has found that AgNPs significantly reduced respiratory syncytial virus (RSV) infection by directly inactivating RSV through binding to virions (Yang et al. 2016, 2017). The research conducted by Chikte (Chikte et al. 2019) also demonstrated the rapid bactericidal activity of the synthesized CuNPs against Xanthomonas axonopodis pv. punicae in vitro. In our study, CuONPs exhibited superior inhibitory effects on the virus before plant infection. The presence of CuONPs (10 mg/L and 100 mg/L) in the early stage of viral infection was found to mitigate the pathogenicity of TMV. In the therapeutic effect, tobacco had been infected with TMV before the application of CuONPs. Due to TMVs having infected and replicated in plants, the therapeutic efficacy of CuONPs (10 mg/L) during the later stages of infection proved insufficient for conferring resistance against viral proliferation and accumulation. However, CuONPs (100 mg/L) still had an inhibitory effect on TMV. Menkissoglu’s study revealed that Cu particles can adhere to the leaf surface, forming a protective film that releases toxic Cu2+ ions upon contact with low-pH water (Menkissoglu 1991). Plant exudates have been shown to enhance the solubility and availability of Cu by generating weak acids on the plant surface (Arman and Wain 1958), which may potentially impede the initial infection of TMV by CuONPs. Therefore, the protective effect of CuONPs on plants exhibited greater potency than its therapeutic effect.
The aforementioned speculation may be a part of the reason why CuONPs (100 mg/L) suppressed TMV infection. Pre-application of certain antivirals prior to viral infection in plants could elicit the plant’s innate immune defense response, thereby mitigating virus infection in plants (Reina et al. 2020). Certain biological and abiotic stresses could elicit systemic resistance against pathogens by upregulating the expression of genes associated with plant defense (Cai et al. 2020). Considering that the application of CuONPs (100 mg/L) on leaves induced a cascade of plant responses, it was imperative to acknowledge the consequential physiological alterations in plants themselves. Although the mechanisms of how CuONPs induced plant resistance against viruses were still unclear, plant hormones were crucial in various aspects of plant biology, such as defense against pathogens (Islam et al. 2019). Among the numerous phytohormones, SA, ET, ABA, and JA assumed pivotal roles in orchestrating plant immunity and fortifying against exogenous stresses (Spoel and Dong 2008; Imada et al. 2016). Our findings demonstrated that the antiviral efficacy of CuONPs primarily stems from their ability to induce plant hormone-mediated resistance, as evidenced by the upregulation of several defense marker genes (Fig. 5). The application of CuONPs induced the activation of SA, ET, and JA related genes, thereby facilitating the enhancement of plant resistance. It is widely acknowledged that modulating specific plant hormones to augment plant resistance constitutes a crucial aspect of the plant defense response (Chen et al. 2013).
A previous study also demonstrated that the combined application of CuONPs and Bacillus subtilis could enhance growth parameters in wheat, including root length, stem length, and biomass (Haider et al. 2023). Recently, a study also showed that CuONPs could promote the growth of nutrients (El-Sayed et al. 2023). Another study found that CuONPs were effective under greenhouse conditions, which could reduce disease incidence and increase biomass and yield (Elmer et al. 2018). The results of our study, in conjunction with these findings, demonstrated that a specific dosage of CuONPs (100 mg/L) exhibited plant growth promotion, which indicated negligible phytotoxicity of CuONPs in plants. Consequently, our research proposed that the application of 100 mg/L CuONPs could confer plant protection and enhance agricultural productivity.
In conclusion, the application of CuONPs (100 mg/L), derived from orange peel through biosynthesis technology, exhibited significant inhibition against TMV infection in plants and promoted plant growth. The application of CuONPs (100 mg/L) exhibited a significant inhibiting effect on TMV infection. We further proposed that CuONPs inhibited TMV by destructing virion and enhancing the disease resistance-related hormone signaling pathways of SA, JA, and ET. On the leaf surface, the presence of CuONPs attenuated the infectivity of TMV particles, thereby enhancing the protective efficacy. The enhancement of resistance and growth promotion in tobacco by CuONPs may be attributed to the effect of different time points. Overall, this study presents a promising and plant-friendly approach for controlling TMV, which can be extrapolated to managing other plant virial diseases. Furthermore, our findings suggest that CuONPs have the potential to inhibit other plant virial diseases by directly poisoning pathogens by destructing the structure of the pathogen and indirectly inhibiting the pathogen by enhancing plant immunity.
Conclusion
CuONPs, which were biosynthesized by orange peel extract in our study, could control TMV disease by directly destroying the structure of viral particles and enhancing plant immunity with plant growth promotion. Interactions among CuONPs, TMV, and the host plant are shown in Fig. 7. Both the pre-inoculation and post-inoculation foliar application of CuONPs proved to be effective for controlling TMV. In addition, after the leaves were exposed to the TMV particles, the NPs strongly perturbed the essential infection process of the virus, primarily because the CuONPs directly adhered to the negatively charged TMV, resulting in TMV structural breakage. In conclusion, CuONPs (100 mg/L) demonstrate promising potential as a novel antiviral agent and growth promoter alternative.
Methods
Biosynthesis and characterization of CuONPs
CuONPs were synthesized using the biosynthesis method, according to previous reports (Tshireletso et al. 2021). CuONPs were synthesized by orange peel with copper nitrate (CuNO3)2·5H2O as a precursor. Briefly, fresh orange peel was washed with distilled water and dried in a drying oven for 3 d at 60℃. Then, the orange peel was mashed in a masher and ground into powder. A total of 8 g of orange peel powder was added into 300 mL distilled water and stirred magnetically at 100 °C for 30 min. Next, the supernatant was centrifuged, and the color of the extract solution was orange. Then, Cu(NO3)2·5H2O was added to the extract solution until the solution changed from orange to green. Finally, 1 M NaOH solution was added, and green precipitates were observed, indicating the formation of CuONPs.
The morphology and structure of CuONPs were observed by SEM and TEM. The surface composition and bonding of CuONPs were determined by XPS. The crystal structure of CuONPs was determined by XRD. Qualitative and quantitative analysis of CuONPs was performed by FTIR.
Extraction of TMV
TMV (TMV-GFP) was extracted as described previously (Ghodoum Parizipour and Shahriari 2020; Angga et al. 2022). A total of 40 g of TMV-infected N. benthamiana leaves was ground into powder with liquid nitrogen. The powder was mixed with 200 mL of 0.1 M potassium phosphate buffer (PBS, pH = 7) containing 1% β-mercaptoethanol and stirred on ice for 15 min. Crude virion was obtained by filtration through gauze. Chloroform and n-butanol (1:1) were added into the crude virion, stirred on ice for 15 min, and then centrifuged at 4 °C and 13,201 g for 15 min. The supernatant was transferred to a new centrifuge tube. Then, 8 g of polyethylene glycol (PEG) 6000 was gradually added into the centrifuge tube on the ice. After the PEG was completely dissolved, centrifugation was performed as described above. Subsequently, the supernatant was discarded, and the particles were re-suspended in 43 mL 0.05 M PBS (pH = 7) and incubated at 4°C for 1 h. The mixture was then centrifuged at 13,201 g for 15 min. The supernatant was taken to isolate virus particles by adding 4% NaCl and 4% PEG6000, which was centrifuged at 9168 g for 15 min. Next, the particles were dissolved in 4.3 mL 0.05 M PBS (pH = 7) and centrifuged at 9168 g for 5 min. Finally, the supernatant containing TMV virion was stored in the refrigerator at -20℃.
Plant cultivation
Seeds of N. benthamiana were planted in matrix medium (Pindstrup Mosebrug A/S, Denmark) and germinated under a photoperiod of 16 h at a temperature of 25℃ ± 3℃ and a relative humidity of 50%. Tobacco plants were transplanted when they had two real leaves. Four weeks later, tobacco plants with 5–6 identical growing leaves were selected for the next experiment.
TMV inoculation
A total of 100 µL TMV was inoculated on N. benthamiana by friction. The fluorescence intensity of the leaves was observed under the UV lamp (Blak-Ray B-100AP, Upland, CA). The effects of different treatments on virus proliferation were further evaluated by observing the fluorescence intensity changes in tobacco leaves.
Destruction of TMV particles by CuONPs
Previous studies have found that 100 mg/L CuONPs had a strong destructive effect on pathogenic bacteria in vitro (Shah et al. 2022). Herein, we studied the interaction of CuO (100 mg/L) and CuONPs (100 mg/L) on TMV. TMV particles were mixed with CuO (100 mg/L) and CuONPs (100 mg/L), and deionized water was used as a blank control group. All mixtures were treated for 2 h in vitro at 25℃. The effects of different treatments on TMV particles were observed by TEM.
Antiviral effect of CuONPs in vitro
The high concentrations of CuONPs (500 mg/L) were toxic to plants (Liu et al. 2017), and high concentrations (500 mg/L and 1000 mg/L) of CuONPs caused adverse effects on plant growth during the whole life cycle (Peng et al. 2017). Therefore, 10 mg/L and 100 mg/L were selected. Different concentrations of CuO and CuONPs suspensions (10 mg/L and 100 mg/L) were configured, and deionized water was used as a blank control group. TMV particles were thoroughly mixed with the test materials and incubated at 25℃ for 2 h. Then, the virus was inoculated onto the leaves. The fluorescence intensity of leaves in different treatment groups was observed after 2 dpi. After 5 dpi, the TMV infection of new leaves was observed (Fig. 8a). The experiment was independently repeated three times.
Antiviral effect of CuONPs in vivo
Protection effect
Different concentrations of CuO and CuONPs were sprayed on the tobacco leaves, respectively. The control group was treated with an equal volume of deionized water. After 24 h, tobacco leaves were inoculated with TMV. The fluorescence intensity of inoculated leaves in different treatment groups was observed at 2 dpi. TMV-infected leaves were observed at 5 dpi (Fig. 8b). The experiment was independently repeated three times.
Therapy effect
The tobacco leaves were inoculated with TMV after they were respectively sprayed with different concentrations of CuO and CuONPs 24 h. Virus infection was observed on day 1 and virus proliferation on day 4 (Fig. 8c). All experiments were independently replicated three times.
Anti-virus activity analysis
Quantitative real-time PCR
The expression of TMV was quantified as described previously (Hao et al. 2018; Zhang et al. 2020). TMV expression was determined by quantitative polymerase chain reaction (qPCR). RT-qPCR was performed on an LC480 instrument (Roche, CA, USA) 96-well plate using SYBR® Premix Ex Taq™ (TaKaRa, Guangzhou, China). Total RNA was separated from TMV-infected leaves for cDNA synthesis (Zhang et al. 2020). The 20 µL real-time PCR volume consisted of 10 µL 2×SYBR Premix Ex Taq II, 1 µL primer F (5 µmol/L), 1 µL primer R (5 µmol/L), 1.5 µL cDNA, and 6.5 µL ddH2O.
Expression of defense-related plant hormone genes
Primers of SA, ET, JA, and ABA for the expression of related genes were designed according to previous studies (Kosugi and Ohashi 2000; Liu et al. 2016; **a et al. 2018). RNA was extracted from 0.1 g tobacco leaves of different treatments and then reverse-transcribed into cDNA. The expression levels of SA, ET, JA, and ABA key genes were determined by qPCR.
Western blot analysis of protein
Protein was extracted from 0.1 g leaves using Plant Cell lysis buffer for Western and IP Kits (Beyotime Biotechnology, China). 12% SDS-PAGE was used for gel electrophoresis. The gel was run for 20 min at 90 V and then for 90 min at 110 V. Protein gel electrophoresis (1 h at 100 V) was transferred from the gel to a PVDF membrane for western blot analysis. After electrophoretic transfer, PVDF membranes were washed 3 times with TBST. Nonspecific binding of the antibody was blocked with TBST containing 5% skim milk powder (**ao et al. 2012). The membrane was incubated with primary anti-GFP antibody overnight at 4℃. PVDF was washed with TBST twice for 5 min each time. The membrane was incubated with a secondary antibody (goat anti-rabbit immunoglobulin diluted 1:8000 in TBST plus 1% nonfat dried milk) at room temperature for 2 h. After washing with TBST for 3 times, the immune response sites of the membrane were detected by Super ECL Plus Western Blotting Substrate (Baoguang Biotechnology, China). At the same time, the corresponding SDS-PAGE gel was stained with coomassie bright blue.
Effect of CuONPs on tobacco growth
Different concentrations of CuO and CuONPs were evenly sprayed on the leaves of the whole tobacco plant on the first day. After 12 d, tobacco plant height, fresh weight, dry weight (Kurum et al. 2013), and chlorophyll content were determined (Cubas et al. 2008). Three treatment groups were set up. The experiment was independently repeated three times.
Availability of data and materials
Not applicable.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- ABA:
-
Abscisic acid
- CuONPs:
-
Copper oxide nanoparticles
- dpi:
-
Days post-inoculation
- ET:
-
Ethylene
- FTIR:
-
Fourier transform infrared spectroscopy
- INPs:
-
Inorganic nanoparticles
- JA:
-
Jasmonic acid
- N. benthamiana :
-
Nicotiana benthamiana
- NPs:
-
Nanoparticles
- PVY:
-
Potato Virus Y
- qPCR:
-
Quantitative polymerase chain reaction
- RSV:
-
Respiratory syncytial virus
- SAR:
-
Systemic acquired resistance
- SEM:
-
Scanning electron microscopy
- SA:
-
Salicylic acid
- TMV:
-
Tobacco mosaic virus
- ToMV:
-
Tomato Mosaic Virus
- TuMV:
-
Turnip mosaic virus
- TEM:
-
Transmission electron microscopy
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
References
Angga MS, Malla B, Raya S, Kitano A, **e X, Saitoh H, et al. Development of a magnetic nanoparticle-based method for concentrating SARS-CoV-2 in wastewater. Sci Total Environ. 2022;848:157613. https://doi.org/10.1016/j.scitotenv.2022.157613.
Arman P, Wain RL. Studies upon the copper fungicides. Ann Appl Biol. 1958;46:366–74. https://doi.org/10.1111/j.1744-7348.1958.tb02217.x.
Bos L. 100 years of virology: from vitalism via molecular biology to genetic engineering. Trends Microbiol. 2000;8:82–7. https://doi.org/10.1016/s0966-842x(99)01678-9.
Cai L, Liu CY, Fan GJ, Liu CL, Sun XC. Preventing viral disease by ZnONPs through directly deactivating TMV and activating plant immunity in Nicotiana Benthamiana. Environ Sci-Nano. 2019;6:3653–69. https://doi.org/10.1039/c9en00850k.
Cai L, Zhang W, Jia H, Feng H, Wei X, Chen H, et al. Plant-derived compounds: a potential source of drugs against Tobacco mosaic virus. Pestic Biochem Phys. 2020;169:104589. https://doi.org/10.1016/j.pestbp.2020.104589.
Chakraborty N, Banerjee J, Chakraborty P, Banerjee A, Chanda S, Ray K, et al. Green synthesis of copper/copper oxide nanoparticles and their applications: a review. Green Chem Lett Rev. 2022;15:187–215. https://doi.org/10.1080/17518253.2022.2025916.
Chen Q, Wei T. Cell Biology During Infection of Plant Viruses in Insect Vectors and Plant Hosts. Mol Plant Microbe In. 2020;33:18–25. https://doi.org/10.1094/MPMI-07-19-0184-CR.
Chen Z, Meng H, **ng G, Chen C, Zhao Y, Jia G, et al. Acute toxicological effects of copper nano-particles in vivo. Toxicol Lett. 2006;163:109–20. https://doi.org/10.1016/j.toxlet.2005.10.003.
Chen S-S, Lin J, Chen Q-B, Liu Y-F, Ma L, Li C-J, et al. Quantitative estimation of the effects of propionylshikonin on the binding of TMV RNA and tobacco mRNA to wheat germ ribosome in vitro. Pestic Biochem Phys. 2012;103:135–43. https://doi.org/10.1016/j.pestbp.2012.04.010.
Chen L, Zhang L, Li D, Wang F, Yu D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. P Natl Acad Sci USA. 2013;110:E1963-71. https://doi.org/10.1073/pnas.1221347110.
Chikte RG, Paknikar KM, Rajwade JM, Sharma J. Nanomaterials for the control of bacterial blight disease in pomegranate: quo vadis? Appl Microbiol Biot. 2019;103:4605–21. https://doi.org/10.1007/s00253-019-09740-z.
Cubas C, Gloria Lobo M, González M. Optimization of the extraction of chlorophylls in green beans (Phaseolus vulgaris L.) by N,N-dimethylformamide using response surface methodology. J Food Compos Anal. 2008;21:125–33. https://doi.org/10.1016/j.jfca.2007.07.007.
Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep. 2017;15:11–23. https://doi.org/10.1016/j.btre.2017.03.002.
El-Dougdoug NK, Bondok A, El-Dougdoug KA. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Mid E J Appl Sci. 2018;1:100–11. http://www.curresweb.com/mejas/mejas/2018/100-111.pdf.
El-Sayed E-SR, Mohamed SS, Mousa SA, El-Seoud MAA, Elmehlawy AA, Abdou DAM. Bifunctional role of some biogenic nanoparticles in controlling wilt disease and promoting growth of common bean. AMB Express. 2023;13:41. https://doi.org/10.1186/s13568-023-01546-7.
Elmer WH, White JC. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ Sci Nano. 2016;3:1072–9. https://doi.org/10.1039/C6EN00146G.
Elmer W, De La Torre-Roche R, Pagano L, Majumdar S, Zuverza-Mena N, Dimkpa C, et al. Effect of Metalloid and Metal Oxide nanoparticles on Fusarium Wilt of Watermelon. Plant Dis. 2018;102:1394–401. https://doi.org/10.1094/PDIS-10-17-1621-RE.
Farooq T, Adeel M, He Z, Umar M, Shakoor N, Da Silva W, et al. Nanotechnology and plant viruses: an emerging Disease Management Approach for resistant pathogens. ACS Nano. 2021;15:6030–7. https://doi.org/10.1021/acsnano.0c10910.
George FJ. The early history of copper fungicides. Agric Hist. 1935:9:67–79. https://www.jstor.org/stable/3739659.
Ghodoum Parizipour MH, Shahriari AG. Investigation of antiviral potential of licorice (Glycyrrhiza Glabra L.) Crude Extract against Tobacco Mosaic Virus. J Anim Plant Sci. 2020;30:107–14. https://doi.org/10.36899/japs.2020.1.0013.
Haider HI, Zafar I, Ain QU, Noreen A, Nazir A, Javed R, et al. Synthesis and characterization of copper oxide nanoparticles: its influence on corn (Z. mays) and wheat (Triticum aestivum) plants by inoculation of Bacillus subtilis. Environ Sci Pollut Res Int. 2023;30:37370–85. https://doi.org/10.1007/s11356-022-24877-7.
Hao Y, Yuan W, Ma C, White JC, Zhang Z, Adeel M, et al. Engineered nanomaterials suppress turnip mosaic virus infection in tobacco (Nicotiana Benthamiana). Environ Sci Nano. 2018;5:1685–93. https://doi.org/10.1039/c8en00014j.
Hoseinzadeh E, Makhdoumi P, Taha P, Hossini H, Stelling J, Kamal MA, et al. A review on Nano-Antimicrobials: Metal nanoparticles, methods and mechanisms. Curr Drug Metab. 2017;18:120–8. https://doi.org/10.2174/1389200217666161201111146.
Imada K, Sakai S, Kajihara H, Tanaka S, Ito S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016;65:551–60. https://doi.org/10.1111/ppa.12443.
Islam W, Qasim M, Noman A, Tayyab M, Chen S, Wang L. Management of Tobacco Mosaic Virus through Natural metabolites. Rec Nat Prod. 2018;12:403–15. https://doi.org/10.25135/rnp.49.17.10.178.
Islam W, Naveed H, Zaynab M, Huang Z, Chen HYH. Plant defense against virus diseases; growth hormones in highlights. Plant Signal Behav. 2019;14:e1596719. https://doi.org/10.1080/15592324.2019.1596719.
Jadhav K, Deore S, Dhamecha D, Rajeshwari HR, Jagwani S, Jalalpure S, et al. Phytosynthesis of silver nanoparticles: characterization, Biocompatibility studies, and Anticancer Activity. ACS Biomater Sci Eng. 2018;4:892–9. https://doi.org/10.1021/acsbiomaterials.7b00707.
Jadoun S, Arif R, Jangid NK, Meena RK. Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett. 2020;19:355–74. https://doi.org/10.1007/s10311-020-01074-x.
Kanakari E, Dendrinou-Samara C. Fighting Phytopathogens with Engineered Inorganic-based nanoparticles. Materials. 2023;16:2388. https://doi.org/10.3390/ma16062388.
Kosugi S, Ohashi Y. Cloning and DNA-binding properties of a tobacco Ethylene-Insensitive3 (EIN3) homolog. Nucleic Acids Res. 2000;28:960–7. https://doi.org/10.1093/nar/28.4.960.
Kurum R, Ulukapi K, Aydinsakir K, Onus AN. The influence of Salinity on Seedling Growth of some pumpkin varieties used as Rootstock. Not Bot Horti Agrobo. 2013;41:219–25. https://doi.org/10.15835/nbha4118349.
Liu SL, Wu J, Zhang P, Hasi G, Huang Y, Lu J, et al. Response of phytohormones and correlation of SAR signal pathway genes to the different resistance levels of grapevine against Plasmopara viticola infection. Plant Physiol Bioch. 2016;107:56–66. https://doi.org/10.1016/j.plaphy.2016.05.020.
Liu J, Dhungana B, Cobb GP. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ Toxicol Chem. 2017;37:11–20. https://doi.org/10.1002/etc.3945.
Menkissoglu O. Relationship of free ionic copper and toxicity to Bacteria in solutions of Organic compounds. Phytopathology. 1991;81:1258–63. https://doi.org/10.1094/Phyto-81-1258.
Okpara EC, Fayemi OE. Comparative study of spectroscopic and cyclic voltammetry properties of CuONPs from citrus peel extracts. Mater Res Express. 2019;6:10. https://doi.org/10.1088/2053-1591/ab3abb.
Peng C, Xu C, Liu Q, Sun L, Luo Y, Shi J. Fate and Transformation of CuO nanoparticles in the soil–rice system during the life cycle of Rice plants. Environ Sci Technol. 2017;51:4907–17. https://doi.org/10.1021/acs.est.6b05882.
Rajwade JM, Chikte RG, Paknikar KM. Nanomaterials: new weapons in a crusade against phytopathogens. Appl Microbiol Biot. 2020;104:1437–61. https://doi.org/10.1007/s00253-019-10334-y.
Reina G, Peng S, Jacquemin L, Andrade AF, Bianco A. Hard nanomaterials in time of viral pandemics. ACS Nano. 2020;14:9364–88. https://doi.org/10.1021/acsnano.0c04117.
Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K. Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioproc Biosyst Eng. 2012;35:637–43. https://doi.org/10.1007/s00449-011-0646-4.
Sarkar J, Ghosh M, Mukherjee A, Chattopadhyay D, Acharya K. Biosynthesis and safety evaluation of ZnO nanoparticles. Bioproc Biosyst Eng. 2014;37:165–71. https://doi.org/10.1007/s00449-013-0982-7.
Sarkar J, Mollick MM, Chattopadhyay D, Acharya K. An eco-friendly route of γ-Fe2O3 nanoparticles formation and investigation of the mechanical properties of the HPMC-γ-Fe2O3 nanocomposites. Bioproc Biosyst Eng. 2017;40:351–9. https://doi.org/10.1007/s00449-016-1702-x.
Scholthof KB, Adkins S, Czosnek H, Palukaitis P, Jacquot E, Hohn T, et al. Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol. 2011;12:938–54. https://doi.org/10.1111/j.1364-3703.2011.00752.x.
Shah IH, Ashraf M, Khan AR, Manzoor MA, Hayat K, Arif S, et al. Controllable synthesis and stabilization of Tamarix aphylla-mediated copper oxide nanoparticles for the management of Fusarium wilt on musk melon. 3 Biotech. 2022;12:128. https://doi.org/10.1007/s13205-022-03189-0.
Siddiqui H, Parra MR, Qureshi MS, Malik MM, Haque FZ. Studies of structural, optical, and electrical properties associated with defects in sodium-doped copper oxide (CuO/Na) nanostructures. J Mater Sci. 2018;53:8826–43. https://doi.org/10.1007/s10853-018-2179-6.
Sisira S, Hithisha KS, Syama Sankar J, Nazirin N, Vimalraj RK, Kalaimathi M. Facile synthesis and optimization of CuONPs using Illicium verum & Polianthes tuberosa and their anticancer activity. Inorg Chem Commun. 2022;145:109961. https://doi.org/10.1016/j.inoche.2022.109961.
Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–51. https://doi.org/10.1016/j.chom.2008.05.009.
Tshireletso P, Ateba CN, Fayemi OE. Spectroscopic and Antibacterial properties of CuONPs from Orange, Lemon and Tangerine Peel extracts: potential for combating bacterial resistance. Molecules. 2021;26:586. https://doi.org/10.3390/molecules26030586.
Vargas-Hernandez M, Macias-Bobadilla I, Guevara-Gonzalez RG, Rico-Garcia E, Ocampo-Velazquez RV, Avila-Juarez L, et al. Nanoparticles as potential antivirals in Agriculture. Agriculture. 2020;10:444. https://doi.org/10.3390/agriculture10100444.
Vinothkanna A, Mathivanan K, Ananth S, Ma Y, Sekar S. Biosynthesis of copper oxide nanoparticles using Rubia cordifolia bark extract: characterization, antibacterial, antioxidant, larvicidal and photocatalytic activities. Environ Sci Pollut R. 2022;30:42563–74. https://doi.org/10.1007/s11356-022-18996-4.
Vrandecic K, Cosic J, Ilic J, Ravnjak B, Selmani A, Galic E, et al. Antifungal activities of silver and selenium nanoparticles stabilized with different surface coating agents. Pest Manag Sci. 2020;76:2021–9. https://doi.org/10.1002/ps.5735.
**a X-C, Hu Q-Q, Li W, Chen Y, Han L-H, Tao M et al. Cotton (Gossypium hirsutum) JAZ3 and SLR1 function in jasmonate and gibberellin mediated epidermal cell differentiation and elongation. Plant Cell Tissue Organ Cult. 2018;133:249–62. https://doi.org/10.1007/s11240-018-1378-9.
**ao X, Wu H, Zhou X, Xu S, He J, Shen W, et al. The combination of quantitative PCR and Western blot detecting CP4-EPSPS component in Roundup Ready soy plant tissues and commercial soy-related foodstuffs. J Food Sci. 2012;77:C603–8. https://doi.org/10.1111/j.1750-3841.2012.02718.x.
Yang Y, Klessig DF. Isolation and characterization of a tobacco mosaic virus-inducible myb oncogene homolog from tobacco. Proc Natl Acad Sci USA. 1996;93:14972–7. https://doi.org/10.1073/pnas.93.25.14972.
Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040–8. https://doi.org/10.1039/c5nr07918g.
Yang XX, Li CM, Li YF, Wang J, Huang CZ. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–92. https://doi.org/10.1039/c7nr06520e.
Zhang D, Li J, Li B, Li C, Chen X, Ouyang K. Internal reference gene selection under different hormone stresses in multipurpose timber yielding Tree Neolamarckia cadamba. Forests. 2020;11:1014. https://doi.org/10.3390/f11091014.
Zhu J, Uliana A, Wang J, Yuan S, Li J, Tian M, et al. Elevated salt transport of antimicrobial loose nanofiltration membranes enabled by copper nanoparticles via fast bioinspired deposition. J Mater Chem A. 2016;4:13211–22. https://doi.org/10.1039/c6ta05661j.
Acknowledgements
We thank Prof. Yule Liu (Tsinghua University) for kindly providing the TMV-GFP.
Funding
This work was supported by the Scientific Research Project of Higher Education Department of Guizhou Province (Youth Project) (2022-116), Guizhou Provincial Basic Research Program (Natural Science) (ZK[2023]-096), the science and technology projects of Zunyi Tobacco Company of Guizhou Province (2022XM08), Guizhou University Natural Science Special Post Special Fund (2021-42), and China Tocacco Sichuan Industrial Co., LTD (10202317BA500).
Author information
Authors and Affiliations
Contributions
SL conceived the idea, wrote the manuscript and revised the manuscript. LC, DZ, and XW supervised the project and contributed significantly to analysis. WT, ZL, KY, WD, and SC provided experimental space and technical assistance. SC and SL performed the experiments, XW analyzed the data and figures.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Tian, W., Liu, Z. et al. Biosynthesis of cupric oxide nanoparticles: its antiviral activities against TMV by directly destroying virion and inducing plant resistance. Phytopathol Res 6, 30 (2024). https://doi.org/10.1186/s42483-024-00250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42483-024-00250-z