Abstract
Background
Some studies have suggested the HLA-B27 gene may protect against some infections, as well as it could play a benefit role on the viral clearance, including hepatitis C and HIV. However, there is lack of SARS-CoV-2 pandemic data in spondyloarthritis (SpA) patients.
Aim
To evaluate the impact of HLA-B27 gene positivity on the susceptibility and severity of COVID-19 and disease activity in axial SpA patients.
Methods
The ReumaCoV-Brasil is a multicenter, observational, prospective cohort designed to monitor immune-mediated rheumatic diseases patients during SARS-CoV-2 pandemic in Brazil. Axial SpA patients, according to the ASAS classification criteria (2009), and only those with known HLA-B27 status, were included in this ReumaCov-Brasil’s subanalysis. After pairing them to sex and age, they were divided in two groups: with (cases) and without (control group) COVID-19 diagnosis. Other immunodeficiency diseases, past organ or bone marrow transplantation, neoplasms and current chemotherapy were excluded. Demographic data, managing of COVID-19 (diagnosis, treatment, and outcomes, including hospitalization, mechanical ventilation, and death), comorbidities, clinical details (disease activity and concomitant medication) were collected using the Research Electronic Data Capture (REDCap) database. Data are presented as descriptive analysis and multiple regression models, using SPSS program, version 20. P level was set as 5%.
Results
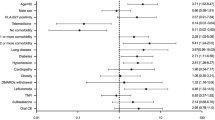
From May 24th, 2020 to Jan 24th, 2021, a total of 153 axial SpA patients were included, of whom 85 (55.5%) with COVID-19 and 68 (44.4%) without COVID-19. Most of them were men (N = 92; 60.1%) with mean age of 44.0 ± 11.1 years and long-term disease (11.7 ± 9.9 years). Regarding the HLA-B27 status, 112 (73.2%) patients tested positive. There were no significant statistical differences concerning social distancing, smoking, BMI (body mass index), waist circumference and comorbidities. Regarding biological DMARDs, 110 (71.8%) were on TNF inhibitors and 14 (9.15%) on IL-17 antagonists. Comparing those patients with and without COVID-19, the HLA-B27 positivity was not different between groups (n = 64, 75.3% vs. n = 48, 48%, respectively; p = 0.514). In addition, disease activity was similar before and after the infection. Interestingly, no new episodes of arthritis, enthesitis or extra-musculoskeletal manifestations were reported after the COVID-19. The mean time from the first symptoms to hospitalization was 7.1 ± 3.4 days, and although the number of hospitalization days was numerically higher in the B27 positive group, no statistically significant difference was observed (5.7 ± 4.11 for B27 negative patients and 13.5 ± 14.8 for B27 positive patients; p = 0.594). Only one HLA-B27 negative patient died. No significant difference was found regarding concomitant medications, including conventional or biologic DMARDs between the groups.
Conclusions
No significant difference of COVID-19 frequency rate was observed in patients with axial SpA regarding the HLA-B27 positivity, suggesting a lack of protective effect with SARS-CoV-2 infection. In addition, the disease activity was similar before and after the infection.
Trial registration
This study was approved by the Brazilian Committee of Ethics in Human Research (CONEP), CAAE 30186820.2.1001.8807, and was registered at the Brazilian Registry of Clinical Trials – REBEC, RBR-33YTQC. All patients read and signed the informed consent form before inclusion.
Similar content being viewed by others
Introduction
Patients with immune-mediated rheumatic diseases (IMRD) have higher risk of infections, particularly associated with immunossuppresion and disease activity. However, patients with spondyloarthritis (SpA) have no increased risk for severe infection, even after exposing to the TNF (TNFi) and IL17 inhibitors (IL17i) [1], differently of individuals with systemic erythematous lupus (SLE) and rheumatoid arthritis (RA) [1,2,3,4]. Some aspects could be highlighted to try to explain these findings when the IMRDs are compared, including lower use of glucocorticosteroids and other immunosuppressive drugs [Statistical analysis To characterize the patient profile, the frequencies percentage and mean and standard deviation (SD) of variables were calculated. Comparisons of means between two groups were performed using the Student’s t test for independent samples. To verify normality data, it was applied the Kolmogorov-Smirnov test. In case normality violation, it was used the Mann-Whitney non-parametric test. The chi-square association test was used to assess the association among categorical variables with standardized adjusted residual calculation, or Fischer’s exact test for small samples. The final logistic regression model used moderate/ severe forms as dependent variable and appropriate adjustments were performed considering all independent variables that had statistical significance up 10% in the univariate analysis. P value was set as significant if below 5%. Statistical analyzes were performed using the SPSS 20.0 statistical software.
Results
A total of 153 axial SpA patients were included, of whom 85 (55.5%) with COVID-19 and 68 (44.4%) without COVID-19. Most of them were men (N = 92; 60.1%) with mean age of 44.0 ± 11.1 years and long-term disease (11.7 ± 9.9 years), and 112 (73.2%) patients had positivity status for HLA-B27. All patients included were COVID-19 confirmed cases, according to Brazilian Ministry of Healthy recommendations, most of them classified according to lab criterion (80.1%), mostly through RT-PCR (58.5%). Regarding biological DMARDs, 110 (71.8%) were on TNF inhibitors and 14 (9.15%) on IL-17 antagonists. No significant difference was found regarding concomitant medications, including conventional or biologic DMARDs between the groups There were no significant statistically differences concerning social distancing, smoking, BMI, waist circumference and comorbidities. Also, there were no significant difference concerning ASDAS-CRP or ASDAS-ESR, but SpA patients HLA-B27 negative had higher score related to pain and other PROs (patient-reported outcomes) measured though the VAS and BASDAI (Table 1). However, when stratifying the relationship between the disease activity, measured by these variables (VAS and BASDAI), and the COVID-19 severity, no significant differences were observed (Table 2). Interestingly, no new episodes of arthritis, enthesitis or extra-musculoskeletal manifestations were reported after the COVID-19.
Comparing SpA patients with and without COVID-19, the HLA-B27 positivity was not different between groups (n = 64, 75.3% vs. n = 48, 48%, respectively; p = 0.514). Similarly, when comparing mild, moderate, and severe COVID-19, according to the HLA-B27 status, no significant difference was found (Table 3). The mean time from the first symptoms to hospitalization was 7.1 ± 3.4 days, and although the number of hospitalization days has been numerically higher in the B27 positive group than in those negative status, no statistically significant difference was observed (13.5 ± 14.8 vs. 5.7 ± 4.11, respectively; p = 0.594). Only one HLA-B27 negative patient died. The hospitalization frequency, considered as moderate COVID, was higher in the B27 positive patient group (p = 0.031) (Table 3). Disease activity was similar before and after the infection the control group had significantly higher disease activity score, according to ASDAS-CRP (3.8 vs. 3.2, p = 0.08).
Discussion
Our data demonstrated no significant difference of COVID-19 frequency rate or severity pattern in patients with axial SpA regarding the HLA-B27 positivity status, suggesting a lack of protective effect against the SARS-CoV-2 infection. In addition, the disease activity was similar before and after the infection. Our data emphasize that the disease behavior in axial SpA patients is similar to the general population, regardless biologic therapy and HLA-B27 status.
Similarly, Rosenbaum et al. using an online self-reported survey in 3,435 SpA patients from 65 countries (85% with AS; 76.1% were HLA-B27 positive from 2,836 aware of HLA-B27 status) from the Spondylitis Association of America (SAA) between 10 and 2020 and 26 April 2021, showed also similar rate of infection between HLA-B27 positive (1.4%) and negative (1.5%), as well as only in those tested (0,6% in both groups) and without significant difference related to self-reported COVID-19 severity. In addition, they did not find any significant difference the incident rate ratio for COVID-19 compared to the US population adjusted for age and sex, regardless HLA-B27, diagnosis of SpA or its treatment. On the other hand, it is important to note that some potential bias, such as responders’ profile, low number of infected individuals (n = 41), self-reported diagnosis of SpA and HLA-B27 status, as well as COVID-19, including confirmatory lab testing in only 36.9% of sample and severity based on patient’s opinion), and some limitations, such as lack of information regarding the disease activity data. Thus, our prospective cohort study provides more consistent data regarding the relationship between HLA-B27 and COVID-19 susceptibility and severity [33,34,35].
During human evolution, the existence of conserved HLA haplotypes is resulting from a selective pressure by some pathogens that have edited the immune response genes and provided higher survival probability over time. Considering that the virus-specific T cell response is critical for determining the infection severity, as well as viral clearance, interferon release, duration of immunity and efficacy of vaccines, our initial hypothesis was driven to the protective role of HLA-B27 against SARS-CoV-2 infection in axial SpA patients, a similar finding found in other viral infections. However, our hypothesis was not confirmed in this prospective face-to-face analysis [36].
Regarding specifically the SARS-CoV-2, the infectivity, severity, and mortality rates related to COVID-19 have not affected all countries equally, suggesting that several aspects could be involved in different populations. Beyond traditional risk factors, such as age, comorbidities, lack of masking/ social distancing and viral load, some authors highlight the relevance of genetic biomarkers as potential drivers for ethnic susceptibility and unfavorable prognosis [37, 38]. Although the HLA systems have a strong linkage disequilibrium that can result in conserved multi-locus haplotypes depending on how the alleles are arranged in adjacent loci of a specific region of the chromosome, it is the best way to characterize the host genetic variance concerning the innate immunity, especially related to MHC class I, which plays a major role in susceptibility to viral infections [39]. Thus, these aspects may contribute to the difference in mortality among various countries [40]. However, a recent review found heterogeneous findings in the association of inter-individual genetic variants with COVID-19 susceptibility and severity COVID-19, but without any relationship with HLA-B27 [41].
Furthermore, NK and dendritic cells, neutrophils and other antigen presenting cells with different signaling ways, including PD-1 and cytokine storm, and exhausted lymphocytes are involved in the COVID-19 pathophysiology [36]. Recently, it was demonstrated CD8+ T cells derived from SARS-CoV-2 survivors exhibited polyfunctional effector responses to two novel NC-derived peptides identified as HLA-binders, although not specified [36, 42, 43].
Considering previous outbreaks, Lin et al. [44] reported higher risk and severity of SARS-CoV infection in health care workers carrying HLA-B*4601 (risk and severity) and HLA-B*5401 (only risk) in Taiwan [45]. On the other hand, no significant association was observed in Chinese individuals regarding genotypic patterns of HLA-A, -B and -DRB1 loci in SARS patients when compared to a co-resident population. In Saudi individuals, Hajeer at al demonstrated significant association with MHC class II (HLA-DRB1*11:01 and DQB1*02:02) and susceptibility to severe MERS-CoV (Middle East respiratory syndrome) [45, 46].
Regarding the COVID-19, Leite et al. evaluated data sets of HLA-B alleles, KIR genes and functional single nucleotide polymorphisms (SNPs) in cytokines related to COVID-19 cytokine storm and found significant correlation between eight HLA-B alleles and polymorphisms in three cytokine genes (IL6, IL10, and IL12B) and daily death rates across countries [47]. Sakuraba et al. demonstrated significant association between HLA-C*05 and higher COVID-19 mortality in 74 countries, after multiple adjustments. A 1% increase in the allele frequency of HLA-C*05 was associated with an increase of 44 deaths/million. Noteworthy, the combination of HLA-C*05 to its receptor KIR2DS4fl, expressed on NK cells, causes NK cell-induced hyperactive immune response and higher mortality rate. Countries with similar ethnic and/or geographic background responded in a similar pattern to each pandemic [48].
Although the HLA genotypes might affect the susceptibility to SARS-CoV-2 infection or severity of COVID-19, these aspects are not still clear up to present day. Toyoshima et al. identified a total of 1234 mutations by comparing with the reference SARS-CoV-2 sequence after analyzing 12,343 SARS-CoV-2 genome sequences of individuals from 28 countries in six geographic areas. They found that ORF1ab 4715 L and S protein 614G variants and the frequency of several HLA alleles, including HLA-A*11:01, were associated with number of infected cases and not as an independent factor for fatality rate in each country [49].
More recently, Pretti et al., evaluating 140 HLA alleles and the landscape of 3,723 potential HLA-I A and B restricted SARS-CoV-2-derived antigens in 37 countries, added a new information regarding three potential antigens coverage of S and N derived peptides and the number of deaths [50]. Similarly, other authors have also reported same findings related to SARS-CoV-2 immune responses, susceptibility and different genetic backgrounds. Interestingly, Guo et al. found diverse capacities of S protein specific epitope presentation by different HLA alleles with very limited number of predicted epitopes for HLA-B*2705, HLA-B*4402 and HLA-B*4403 and as high as 132 epitopes for HLA-A*6601, suggesting individuals with HLA-B*44 are more prone to SARS-CoV-2 infection. These findings could help us to better understanding the role of HLA-B27 in the COVID-19 [25, 51].
Thus, some points could be highlighted to explain the lack of association between COVID-19 and HLA-B27. Firstly, only Guo study showed some relationship with B27 in general population database across the countries. Secondly, the cytokines pattern and inflammatory signaling ways involved with severe COVID do not seem to be related to Th17 activation, an immune-inflammatory via strongly associated with SpA. Thirdly, the low plasticity of epitopes found by Guo et al. in HLA-B27 could not be crucial to influence the antigen presentation to the spike proteins from SARS-CoV-2 and other coronavirus family. Fourthly, severe COVID-19 have been reported in all IMRDs and the unfavorable outcome has been more related to age and comorbidities than underlying rheumatic disease. On the other hand, the SARS-CoV-2 could work as a trigger to a myriad of musculoskeletal syndromes. Although some authors have reported anecdotal cases of reactive arthritis after COVID-19, the most recent systematic review showed that the manifestations with consistent association with SARS-CoV-2 infection were autoimmune cytopenia, cutaneous vasculitis, encephalitis, and Guillain-Barre syndrome [52]. Considering the inclusion period ended before the massive vaccination against the COVID-19, it is important to mention that our data are related to the SARS-CoV-2 itself and HLA-B27 positivity without any interference of adenovirus vectors, inactivated full virus or mRNA vaccines.
Another important thing to be considered is related to endoplasmatic reticulum aminopeptidases (ERAPs) [53]. As they are pivotal enzymes for trimming peptides and to generate optimal-length antigens to fit into the MHC class I groove, the SARS-CoV-2 likely must not cause any important impairment in this cell step neither related to repertoire of antigens presented by HLA-B27. The inadequate activation of NK and CD8+ T cells could have other pathophysiological mechanisms. Nonetheless, these points are merely speculative because there is no information on the ERAP1, ERAP2, and LNPEP, as well as co-segregation with HLA-B27 subtypes, which are involved in antigen presentation and peptide trimming and the COVID-19 occurrence and severity. Interestingly, it is important to note these multifaceted aminopeptidases regulates the renin–angiotensin system and higher susceptibility to hypertension, other aspects associated with severe COVID-19 [54]. Considering that an increased risk of some comorbidities has been reported in patients with spondyloarthritis, including hypertension and other cardiovascular diseases, more attention could be given to these aspects to understand better this complex interaction among genetic, environmental and several cellular processes [55].
Additionally, the concomitant conventional and biological DMARDs are also related to higher risk of infections, particularly related to immune response impairment against several pathogens, we did not find any significant association with severe COVID-19 in our SpA database, regardless HLA-B27 status. Considering all forms of COVID-19, we observed 19 cases in B27 negative SpA patients and 52 in those B27 positive, suggesting lack of protective role regarding the SARS-CoV-2 infection. However, when evaluating only hospitalized patients (moderate COVID-19), there was a greater number of patients in the B27 negative group. It is important emphasizing that the effect size was very small (OR = 0.13; 95%CI 0.02–0.81) in only 6 patients (4 HLA-B27 negative and 2 HLA-B27 positive), which does not allow us to draw conclusions about that.
To our best knowledge, our database is the largest and robust database regarding SpA patients during COVID-19 pandemic and comparing to SpA patients no infected by SARS-CoV-2. However, our ongoing observational prospective registry has some limitations, such as short period of follow-up (until infection resolution).
In conclusion, our data did not support the protective role of HLA-B27 on SARS-CoV-2 infection, including occurrence, ability to assert immune control of novel coronavirus and COVID-19 severity. More studies are needed to understand the role of HLA-B27 and variants of SARS-CoV-2.
Data Availability
All clinical and laboratory data was collected and stored on the REDCAP (Research Electronic Data Capture) platform, such as demographic data, details of infection and treatment of COVID-19 (diagnosis, test performed, treatment, epidemiology and outcomes including hospitalization, mechanical ventilation and death). Also, comorbidities and clinical characteristics, including disease time, disease activity parameters: BASDAI [Bath Ankylosing Spondylitis Disease Activity Index], ASDAS-CRP and ASDAS-ESR [Ankylosing Spondylitis Disease Activity Score], current drug therapy).
References
Fouque-Aubert A, Jette-Paulin L, Combescure C, Basch A, Tebib J, Gossec L. Serious Infections in patients with ankylosing spondylitis with and without TNF blockers: a systematic review and meta-analysis of randomised placebo-controlled trials. Ann Rheum Dis. 2010;69(10):1756–61.
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of Infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–93.
Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, et al. Risk of Infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15(sup1):11–34.
Hou Lqiong, Jiang G, xue, Chen Y, fei, Yang XM, Meng L, Xue M, et al. The comparative safety of TNF inhibitors in Ankylosing Spondylitis—a Meta-Analysis Update of 14 randomized controlled trials. Clin Rev Allergy Immunol. 2018;54(2):234–43.
Resende GG, Meirelles E, de Marques S, Chiereghin CDL, Lyrio A, **menes AM. The Brazilian society of Rheumatology guidelines for axial spondyloarthritis – 2019. Adv Rheumatol. 2020;60(1):19.
Voruganti A, Bowness P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology. 2020;161(2):94–102.
Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DCO, Sturrock RD. ANKYLOSING SPONDYLITIS AND HL-A 27. The Lancet. 1973;301(7809):904–7.
den Uyl D, van der Horst-Bruinsma IE, van Agtmael M. Progression of HIV to AIDS: a protective role for HLA-B27? AIDS Rev. 2004;6(2):89–96.
Mathieu A, Cauli A, Fiorillo MT, Sorrentino R. HLA-B27 and Ankylosing Spondylitis geographic distribution as the result of a genetic selection induced by Malaria endemic? A review supporting the hypothesis. Autoimmun Rev. 2008;7(5):398–403.
de Paula Rodrigues KF, Faria e Arantes TE, Muccioli C, de Andrade Neto JL, Pinheiro MM. Incidence of toxoplasma retinochoroiditis in patients with ankylosing spondylitis after using TNF-α blockers. Parasitol Int. 2013;62(3):272–5.
Taugor JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, et al. Inflammatory Disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169(1):209–23.
Rosenbaum JT, Davey MP. Time for a gut check: evidence for the hypothesis that HLA-B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum. 2011;63(11):3195–8.
Carter JD, Gérard HC, Espinoza LR, Ricca LR, Valeriano J, Snelgrove J, et al. Chlamydiae as etiologic agents in chronic undifferentiated spondylarthritis. Arthritis Rheum. 2009;60(5):1311–6.
Asquith MJ, Stauffer P, Davin S, Mitchell C, Lin P, Rosenbaum JT. Perturbed mucosal immunity and Dysbiosis Accompany Clinical Disease in a rat model of Spondyloarthritis. Arthritis & Rheumatology. 2016;68(9):2151–62.
Appel H, Kuon W, Kuhne M, Wu P, Kuhlmann S, Kollnberger S, et al. Use of HLA-B27 tetramers to identify low-frequency antigen-specific T cells in Chlamydia-triggered reactive arthritis. Arthritis Res Ther. 2004;6(6):R521.
Colbert RA, Navid F, Gill T. The role of HLA-B*27 in spondyloarthritis. Best Pract Res Clin Rheumatol. 2017;31(6):797–815.
Pennisi M, Perdue J, Roulston T, Nicholas J, Schmidt E, Rolfs J. An overview of reactive arthritis. JAAPA. 2019;32(7):25–8.
Gracey E, Vereecke L, McGovern D, Fröhling M, Schett G, Danese S, et al. Revisiting the gut–joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol. 2020;16(8):415–33.
de Oliveira TL, Libanori HT, Pinheiro MM. Axial spondyloarthritis after bariatric Surgery: a 7-year retrospective analysis. Adv Rheumatol. 2019;59(1):8.
Breban M, Beaufrère M, Glatigny S. The microbiome in spondyloarthritis. Best Pract Res Clin Rheumatol. 2019;33(6):101495.
Mathieu A, Paladini F, Vacca A, Cauli A, Fiorillo MT, Sorrentino R. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune Diseases: a unifying hypothesis. Autoimmun Rev. 2009;8(5):420–5.
Neumann-Haefelin C. HLA-B27-mediated protection in HIV and Hepatitis C virus Infection and pathogenesis in spondyloarthritis. Curr Opin Rheumatol. 2013;25(4):426–33.
Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, et al. Dominant influence of an HLA-B27 restricted CD8 + T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43(3):563–72.
Neumann-Haefelin C, Oniangue-Ndza C, Kuntzen T, Schmidt J, Nitschke K, Sidney J, et al. Human leukocyte antigen B27 selects for rare Escape mutations that significantly impair Hepatitis C virus replication and require compensatory mutations. Hepatology. 2011;54(4):1157–66.
Guo E, Guo H. CD8 T cell epitope generation toward the continually mutating SARS-CoV-2 spike protein in genetically diverse human population: implications for Disease control and prevention. PLoS ONE. 2020;15(12):e0239566.
Marques CDL, Ribeiro SLE, Albuquerque CP, de Sousa Studart SA, Ranzolin A, de Andrade NPB et al. COVID-19 was not associated or trigger Disease activity in spondylarthritis patients: ReumaCoV-Brasil cross-sectional data. Adv Rheumatol. 2022;62(1).
Marques CDL, Kakehasi AM, Pinheiro MM, Mota LMH, Albuquerque CP, Silva CR et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic Diseases and COVID-19: first results of ReumaCoV Brasil registry. RMD Open. 2021;7(1).
Marques C, Kakehasi AM, Gomides APM, Paiva EDS, dos Reis Neto ET, Pileggi GCS, et al. A Brazilian cohort of patients with Immuno-mediated chronic inflammatory Diseases infected by SARS-CoV-2 (ReumaCoV-Brasil Registry): protocol for a prospective, observational study. JMIR Res Protoc. 2020;9(12):e24357.
Brasil. In: Saúde, Md, editors. Boletim epidemiológico - doença pelo coronavírus 2019 - ampliação da vigilância, medidas não farmacológicas e descentralização do diagnóstico laboratorial. Brasil; 2020.
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining Disease status in ankylosing spondylitis: the bath ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–91.
Machado PM, Landewé R, van der Heijde D. Ankylosing Spondylitis Disease Activity score (ASDAS): 2018 update of the nomenclature for Disease activity states. Ann Rheum Dis. 2018;77(10):1539–40.
Rosenbaum JT, Hamilton H, Choi D, Weisman MH, Reveille JD, Winthrop KL. Biologics, spondylitis and COVID-19. Ann Rheum Dis. 2020;79(12):1663–5.
Rosenbaum JT, Hamilton H, Weisman MH, Reveille JD, Winthrop KL, Choi D. The effect of HLA-B27 on susceptibility and severity of COVID-19. J Rheumatol. 2021;48(4):621–2.
Rosenbaum JT, Weisman MH, Hamilton H, Shafer C, Aslanyan E, Howard RA, et al. The interplay between COVID-19 and Spondyloarthritis or its treatment. J Rheumatol. 2022;49(2):225–9.
Bordallo B, Bellas M, Cortez AF, Vieira M, Pinheiro M. Severe COVID-19: what have we learned with the immunopathogenesis? Adv Rheumatol. 2020;60(1):50.
Asrani P, Hasan GM, Sohal SS, Hassan MI. Molecular basis of Pathogenesis of coronaviruses: a Comparative Genomics Approach to Planetary Health to prevent zoonotic outbreaks in the 21st Century. OMICS. 2020;24(11):634–44.
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human Respiratory Disease in China. Nature. 2020;579(7798):265–9.
Slatkin M. Linkage disequilibrium — understanding the evolutionary past and map** the medical future. Nat Rev Genet. 2008;9(6):477–85.
di Maria E, Latini A, Borgiani P, Novelli G. Genetic variants of the human host influencing the coronavirus-associated phenotypes (SARS, MERS and COVID-19): rapid systematic review and field synopsis. Hum Genomics. 2020;14(1):30.
Fricke-Galindo I, Falfán-Valencia R. Genetics Insight for COVID-19 susceptibility and severity: a review. Front Immunol. 2021;12.
Tizaoui K, Zidi I, Lee KH, Ghayda RA, Hong SH, Li H, et al. Update of the current knowledge on genetics, evolution, immunopathogenesis, and transmission for coronavirus Disease 19 (COVID-19). Int J Biol Sci. 2020;16(15):2906–23.
Ebrahimi N, Aslani S, Babaie F, Hemmatzadeh M, Hosseinzadeh R, Joneidi Z, et al. Recent findings on the coronavirus Disease 2019 (COVID-19); immunopathogenesis and immunotherapeutics. Int Immunopharmacol. 2020;89:107082.
Lin M, Tseng HK, Trejaut JA, Lee HL, Loo JH, Chu CC, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus Infection. BMC Med Genet. 2003;4(1):9.
**ong P, Zeng X, Song MS, Jia SW, Zhong MH, **ao LL, et al. Lack of association between HLA-A, -B and -DRB1 alleles and the development of SARS: a cohort of 95 SARS-recovered individuals in a population of Guangdong, southern China. Int J Immunogenet. 2008;35(1):69–74.
Hajeer A, Balkhy H, Johani S, Yousef M, Arabi Y. Association of human leukocyte antigen class II alleles with severe Middle East respiratory syndrome-coronavirus Infection. Ann Thorac Med. 2016;11(3):211.
Leite M, de Gonzalez-Galarza M, Silva FF, Middleton BCC. D, Santos EJM dos. Predictive immunogenetic markers in COVID-19. Hum Immunol. 2021;82(4):247–54.
Sakuraba A, Haider H, Sato T. Population Difference in Allele frequency of HLA-C*05 and its correlation with COVID-19 mortality. Viruses. 2020;12(11):1333.
Toyoshima Y, Nemoto K, Matsumoto S, Nakamura Y, Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65(12):1075–82.
Pretti MAM, Galvani RG, Vieira GF, Bonomo A, Bonamino MH, Boroni M. Class I HLA Allele Predicted restricted antigenic coverages for Spike and Nucleocapsid Proteins Are Associated with deaths related to COVID-19. Front Immunol. 2020;11.
Lee E, Sandgren K, Duette G, Stylianou Vv, Khanna R, Eden JS et al. Identification of SARS-CoV-2 Nucleocapsid and Spike T-Cell epitopes for assessing T-Cell immunity. J Virol. 2021;95(6).
Tang KT, Hsu BC, Chen DY. Autoimmune and rheumatic manifestations Associated with COVID-19 in adults: an updated systematic review. Front Immunol. 2021;12.
Saulle I, Vicentini C, Clerici M, Biasin M. An overview on ERAP roles in Infectious Diseases. Cells. 2020;9(3):720.
Paladini F, Fiorillo MT, Tedeschi V, Cauli A, Mathieu A, Sorrentino R. Ankylosing spondylitis: a Trade off of HLA-B27, ERAP, and Pathogen interconnections? Focus on Sardinia. Front Immunol. 2019;10:35.
Moltó A, Etcheto A, van der Heijde D, Landewé R, van den Bosch F, Bautista Molano W, et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis. 2016;75(6):1016–23.
Acknowledgements
We would like to acknowledge the Brazilian Rheumatology Society for the contribution to this study, providing the necessary support in such difficult times.
Funding
There was no funding for our research.
Author information
Authors and Affiliations
Contributions
Mota GD: The main author, responsible of performing this sub analysis, regarding the role of HLAB27 on COVID19 infection among patients with axial spondyloarthritis. Also, responsible for the data collection in one of the participating centers of the original trial ReumaCoV Brasil. Pinheiro, MM: Project supervisor and a scientific advisor responsible for the design and development of the thesis, also the researcher responsible for data collection in one of the participating centers of the original trial ReumaCoV Brasil. Marques CL, Ribeiro SL, Albuquerque C, Castro G, Fernandino D, Omura F, Ranzolin A, Resende G, Silva N, Souza M, Studart S, Xavier R, Yazbek M: particpating members of the other centers among the ReumaCov trial. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was designed as a sub analysis from a national multicenter study (ReumaCoV-Brazil) with the idealization, support, and supervision of the Brazilian Society of Rheumatology during the emergency period of the COVID-19 pandemic and with the Federal University of Pernambuco as the coordinating center and managing of ethical demands. The central and original project was approved by CONEP (CAAE 30186820.2.1001.8807) on April 5, 2020.
The research team contacted all patients identified by the eligibility criteria by telephone, giving initial authorization and permission for the collection of clinical information. When the restrictions of the pandemic allowed face-to-face consultation in the respective center, they all read and signed the consent form.
As mentioned above, we used the same database of patients that had already been approved by CONEP as an unfolding. We emphasize that there is no need for further collection of clinical or laboratory data because the HLA-B27 positivity and evaluation with the metrics used in the study are part of the follow-up routine of patients with spondyloarthritis.
In addition, it is important to emphasize that the original ReumaCoV-Brazil study was conceived and developed in full pandemic context, with restrictions for face-to-face medical evaluations and logistical difficulties, in view of the sanitary recommendations of social distancing. Thus, many data needed to be collected by telephone and by searching for previous information, retrospectively, in the patient’s electronic medical record (PEP), with prospective updates on the outcomes of the evolution of COVID-19 and disease activity.
Consent for publication
The authors agreed with the publication.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mota, G., Marques, C., Ribeiro, S. et al. HLA-B27 did not protect against COVID-19 in patients with axial spondyloarthritis – data from the ReumaCov-Brasil Registry. Adv Rheumatol 63, 56 (2023). https://doi.org/10.1186/s42358-023-00340-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-023-00340-0