Abstract
Background
Zooplankton exhibit strong responses for minute changes in their habitat. Therefore, this study intended to investigate the impacts of hydro-climatic factors and related water quality variables on zooplankton abundance and diversity in the perennial Vavuniya reservoir (VR) and seasonal Thandikulam reservoir (TR), Vavuniya, Sri Lanka.
Results
Zooplankton diversity and abundance showed some peculiarities between seasonal and perennial reservoirs under varying hydro-climatic factors and water quality parameters during wet and dry seasons (July 2018–February 2019). There were 14 Rotifera, 4 Cladocera and 5 Copepod species in VR, and in TR, 12 Rotifera, 2 Cladocera and 5 Copepod species were identified. Although these reservoirs are located at proximity (2.48 km), the responses via zooplankton community structure toward environmental factors have been shown uniqueness to some extent. Significant negative correlation was observed for increasing \({\text{NO}}_{{3}}^{ - }\) (p = 0.044) and \({\text{PO}}_{{4}}^{{{3} - }}\) (p = 0.010) concentrations with zooplankton diversity in perennial VR; however, only \({\text{PO}}_{{4}}^{{{3} - }}\) concentration showed a significantly (p = 0.042) negative correlation in seasonal TR. Interestingly, total zooplankton abundance showed significant negative correlations with \({\text{NO}}_{{3}}^{ - }\) (p = 0.013) and \({\text{PO}}_{{4}}^{3 - }\) (p = 0.038) in perennial VR; however, insignificant negative correlation was observed in seasonal TR. Copepods make up the majority of total zooplankton abundance in both reservoirs and were significantly affected by increased atmospheric temperature. Cladocera abundance in VR was highly sensitive (p = 0.032; r = − 0.856) to changes in water level. Increasing water level significantly favors the total zooplankton abundance in the seasonal reservoir but not in perennial.
Conclusions
Sensitivity of zooplankton community structure varies between seasonal and perennial reservoirs in different magnitudes. This is due to the increasing temperatures, increasing phosphate levels and decreasing water levels with the combination of other factors resulting in specific responses of zooplankton diversity and abundance. Therefore, it would not be surprised to use zooplankton community structure to sense the vibrant responses of different freshwater habitats.
Similar content being viewed by others
Background
Sri Lanka is a tropical country, highly susceptible to climate change. During 1961–1990, country’s atmospheric temperature increased by about 0.02% and mean annual rainfall decreased by around 7% compared to previous 30 years (Eriyagama et al., 2010). Moreover, these climate changes have ability to alter the natural ecosystems associated with water cycle (Climate Change Secretariat; Ministry of Mahaweli Development & Environment, 2016). Zooplankton are tiny aquatic animal component that are not capable to swim against the water current, act as bridge for aquatic food web and often used as environmental indicators (Jeppesen et al., 2011). Due to their short lifespan, zooplankton community structure can be changed rapidly, and it is identifiable within a short time period with environmental changes (Jeppesen et al., 2011; Tian et al., 2017; Zannatul et al., 2009). Zooplankton community depict various preferences for trophic state and the water quality and are recognized to vary predictably with wetland quality and show a rapid respond to surrounding environmental changes (Riato et al., 2014). Recent limnological studies have been more focused on the climate change consequences and zooplankton responses for those changes (Adrian et al., 2009; Vadadi-Fülöp et al., 2012). Hydro-climatic factors were changing with the climate system and climatic variability greatly affects the water resources (Jun & Bantin, 2017). Perennial reservoirs retain water throughout the year, while seasonal reservoirs tend to dry up in latter dry season annually (Impact of Climate Change on culture-based fisheries in seasonal reservoirs & Sri Lanka, 2012). As a seasonal reservoir, Thandikulam reservoir exhibited noticeable water-level changes with the season during this study. Being a perennial, Vavuniya reservoir water-level fluctuations are not vigorous. The study was aimed to detect the hydro-climatic variables, viz. monthly rainfall RF (mm), water level WL (m) and atmospheric temperature AT (°C) as well the water quality parameters, viz. dissolved oxygen level DO (ppm), nitrate level \({\text{NO}}_{3}^{-}\) (ppm), phosphate level \({\text{PO}}_{4}^{3-}\) (ppm), and water temperature WT (°C) on zooplankton community structure (diversity and abundance) in both reservoirs.
Methods
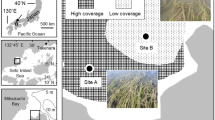
Thandikulam (8° 46′ N, 80° 29′ E) and Vavuniya reservoir (8° 45′ N, 80° 30′ E) are low land dry zone reservoirs, located in Vavuniya district, Northern Province, Sri Lanka (Fig. 1).
Four sampling locations of each reservoir were selected randomly and were demarcated by using geographical positioning system (GPS). In order to cover both dry and wet seasons, the sampling was done from July 2018 to February 2019 fortnightly from just below the water surface (10–15 cm). Zooplankton samples were collected in duplicates by filtering 65 litters of water through 80-µm meshed zooplankton net, and the collected samples were immediately preserved with 5% of formalin. Qualitative analysis was done by using standard guides (Mekong River Commission, 2015), and Shannon–Wiener diversity index (H′) (Shannon & Weiner, 1949) was used to find the diversity (Eq. 1).
where H′ = Shannon–Weiner diversity index, Pi = Fraction of the entire population made up of species I, S = Numbers of species encountered, ∑ = Sum of species 1 to species S.
Quantitative analysis was performed according to Eaton et al. (2005); under compound microscope (Labomed C × L) in low power by using a Sedgwick Rafter cell (Eq. 2).
where n = Total number of plankton in 1 \({\text{m}}^{3}\) (individuals/\({\text{m}}^{3}\)), a = Total number of plankton in 1 ml, C = Volume of concentrate expressed in ml (here it is 10 × \({10}^{ - 3}\)), 1000 = Number of grids in the counting chamber, N = Number of grids employed, L = Volume of water filtered expressed in L (here it is 65 L).
As a result of changing hydro-climatic variables, four major physiochemical parameters were measured during the study, viz. DO, WT, \({\text{NO}}_{{3}}^{ - }\), \({\text{PO}}_{{4}}^{3 - }\). Water temperature was measured from just below the water surface (10–15 cm) by using mercury coiled thermometer. Winkler’s method was used to measure the DO level in water according to US Environmental Protection Agency (1983); \({\text{NO}}_{{3}}^{ - }\) and \({\text{PO}}_{{4}}^{3 - }\) (nutrient) concentration of water was measured by using phenol disulfonic acid method and ascorbic acid-molybdate method, respectively (U.S. Environmental Protection Agency, 1983).
The Pearson correlation matrix (r) was parallelly done for both reservoirs in order to identify the correlations between zooplankton community structures (abundance and diversity) and water quality and hydro-climatic variables by using SPSS 13.0 software.
Results
a) Influence of hydro-climatic and water quality variables on zooplankton species diversity
Total of 23 genera, with 14 Rotifer species, 4 Cladocera species and 5 Copepod, were identified in Vavuniya reservoir (VR), and total of 19 genera with 12 Rotifer species, 2 Cladocera species and 5 Copepod species were observed in Thandikulam reservoir (TR).
Perennial VR showed specific Rotifer species such as Brachionus angularis, B. dimidatus, Keratella cochlearis, Filinia longiseta, Lecane closterocera; Cladocerans such as Ceriodaphnia reticulata, Leptodora kindtii and Copepods such as Tropocyclops sp., while seasonal TR showed unique rotifer species such as B. budapestinensis, Testudinella patina and Lecane bulla.
However, rotifers such as B. angularis, B. calyciflorous, B. caudatus, B. falcatus, B. quadridentatus, B. urceolaris, Keratella tropica, K. valga, Asplanchna sp., Lecane leontina; Cladocera species such as Ceriodaphnia laticaudata, Moina sp.; and Copepoda such as Mesocyclops leukartii, Thermocyclops sp., Calanus sp. were common for both reservoirs (Table 1).
Appendix 01
Water quality parameters, viz. nitrate concentration and phosphate concentrations, showed a significant negative influence on zooplankton diversity compared to other water quality parameters and hydro-climatic variables (Fig. 2 b1, b2).
The \({\text{NO}}_{3}^{-}\) concentration was ranged between 0.225 ppm (February 2019) and 1.003 ppm (August 2018) in VR, whereas 0.001 ppm (December 2018) to 2.609 ppm (August 2018) in TR. \({\text{PO}}_{{4}}^{3 - }\) concentration was fluctuated between 0.002 ppm (July 2018) and 0.189 ppm (August 2018) in VR. It was ranged between 0.002 ppm (December 2018) and 0.286 ppm (August 2018) in TR. Zooplankton diversity showed a significant (p = 0.044) negative correlation with \({\text{NO}}_{3}^{-}\) (r = − 0.820) and \({\text{PO}}_{{4}}^{3 - }\) (p = 0.010; r = − 0.936) in VR, whilst, in TR, there was a significant (p = 0.042) negative correlation (r = − 0.805) between diversity with \({\text{PO}}_{4}^{3-}\) but not with \({\text{NO}}_{3}^{-}\) (Table 2).
Further, AT was ranged between 25 °C (February 2019) and 30.1 °C (July 2018) during the study period. Consequently, water temperature in VR was fluctuated between 24 °C (February 2019) and 27 °C (July 2018), and it was recorded between 25 °C (December 2018; January 2019; February 2019) and 26 °C (July 2018; August 2018) in seasonal TR.
A negative correlation was recorded between zooplankton diversity with increasing atmospheric temperature (Fig. 3a1, a2) and RF (Fig. 3b1, b2) in both reservoirs. Perennial reservoir VR showed a positive relationship between zooplankton diversity with increasing WT (Fig. 3a1). However, negative correlation (Fig. 3 a2) was observed in seasonal TR.
Interestingly, a sudden drop was recorded in DO level in August 2018 and the peak level was recorded in February 2019 in both reservoirs. Numerically it falls between 0.41 ppm and 9.68 ppm in perennial VR, while, in the seasonal TR, the DO level was ranged between 0.41 ppm and 10.09 ppm which was a preferable range for aquatic organisms.
Being a seasonal reservoir, TR showed noticeable fluctuation in WL with the season. 0.3 m depth was recorded in August 2019 (dry season), whereas 2.59 m maximum WL was recorded in January and February 2019 (wet season), while WL in perennial VR was recorded between 2.22 m (July 2018) to 3.71 m (December 2018). Moreover, a positive correlation was detected between zooplankton diversity with increasing WL (Fig. 4 a1, a2) and DO (Fig. 4 b1, b2) in both reservoirs.
b) Influence of hydro-climatic and water quality variables on zooplankton abundance
The total zooplankton abundance was ranged between 2462 individuals/\({\text{m}}^{3}\) (August 2018) and 9538 individuals/\({\text{m}}^{3}\) (February 2019) in VR, while it was ranged between 2461 individuals/\({\text{m}}^{3}\) (July and August 2018)—10,153 individuals/\({\text{m}}^{3}\) (January 2019) in TR (Table 3). Greater Copepoda abundance was recorded followed by Rotifers and Cladocera.
Zooplankton abundance was negatively correlated with AT, WL, \({\text{NO}}_{{3}}^{ - }\) and \({\text{PO}}_{{4}}^{3 - }\) concentrations in VR, while it was positively correlated with DO. However, being a seasonal reservoir, TR exhibits a positive correlation between WL and zooplankton abundance. And there was an inverse relationship between Copepoda zooplankton abundance in TR with temperatures (AT and WT) (Table 4).
Indeed, atmospheric temperature was one of the major hydro-climatic factors. In Vavuniya reservoir, there was a significant (p = 0.012) negative (r = − 0.926) correlation of total zooplankton abundance with AT, especially, Copepoda abundance depicted a significant (p = 0.046) negative correlation (r = − 0.816) with AT. Interestingly, a significant (p = 0.017) negative correlation (r = − 0.905) was recorded between AT and Copepoda abundance in TR as well (Fig. 5 a1, a2).
Moreover, a significant (p = 0.001) positive correlation (r = 0.984) was observed in VR zooplankton abundance with increasing DO (Fig. 6 a1, a2).
When it comes to nutrient loading and zooplankton abundance, interestingly perennial VR showed remarkable significant (p = 0.013) negative correlation (r = − 0.922) between zooplankton abundance and \({\text{NO}}_{3}^{-}\) (Fig. 7 a1).
Simultaneously, there was a significant (p = 0.038) negative correlation (r = − 0.839) with \({\text{PO}}_{{4}}^{{3 - }}\) as well (Fig. 8 a1). As noted earlier, this may due to the algal bloom in both reservoirs.
A significant (p = 0.046) positive (r = 0.817) relationship between WL and total zooplankton abundance in TR was observed in this study. Nevertheless, Cladocera abundance in VR was highly sensitive (p = 0.032; r = − 0.856) to changes in WL (Fig. 9 a1, a2).
A positive correlation was detected in total zooplankton abundance with RF in VR while negative correlation in TR (Fig. 10 a1, a2).
Discussion
Limnological assessments are trending in the current world since the lakes and reservoirs are acting as the biological signals for the environmental changes (Williamson et al., 2009).
Interestingly, the study recognized \({\text{PO}}_{{4}}^{3 - }\) as a determining factor for zooplankton diversity in both perennial and seasonal reservoirs than \({\text{NO}}_{3}^{-}\) concentration. Phosphate concentration is a limiting factor on zooplankton diversity in freshwater bodies (Jeppesen et al., 2011). Anthropogenic activities in VR such as industrial activities, especially rice mill effluents, may attribute to the increased nutrient content in the reservoir. According to Aeriyanie et al. (2021), a high abundance of aquatic plants may also lead to nutrient loading in the reservoirs, especially the phosphate content. Therefore, it can be suggested that major reason of nutrient loading in TR could be due the decomposition of its high abundance of submerged aquatic plants. Furthermore, Adrian et al. (2009) state that increased water temperature also can lead to phosphorous accumulation in lakes. Sangakkara and Wijeyaratne (2015) revealed that the dry season in Sri Lanka experienced a high nutrient (\({\text{NO}}_{{3}}^{ - }\) and \({\text{PO}}_{{4}}^{3 - }\)) loading in reservoirs due to the high evaporation, which may lead to algal bloom, which have the ability to block the filter feeding apparatus of zooplankton, especially in Cladocera species. Ultimately it may induce eutrophication in the reservoirs. And eutrophication negatively influences on the zooplankton diversity in the lakes (Adrian et al., 2009).
Noticeably, zooplankton diversity was declined with increasing \({\text{PO}}_{{4}}^{3 - }\) concentration in both reservoirs (Fig. 2 b1, b2). Bockwoldt et al. (2017) provided a deeper insight to relate the influence of \({\text{PO}}_{{4}}^{3 - }\) concentration on zooplankton diversity in freshwater bodies. According to his records, cyanobacteria density shows an inverse relationship with zooplankton as well as with phytoplankton diversity. Additionally, increased \({\text{PO}}_{{4}}^{3 - }\) concentrations are a stimulus for cyanobacterial blooms. Since cyanobacteria are toxic and less palatable, zooplankton community may undergo food scarcity; which may ultimately reduce their growth, abundance and diversity (Bockwoldt et al., 2017).
Furthermore, Zooplankton diversity variation was observed with RF, WL, AT, DO and WT in each reservoir. Dissolved oxygen also was a crucial factor for zooplankton community structure. According to the studies done by Banerjee et al. (2019); the critical minimum value for the DO in a reservoir is 4.5 ppm. The present study noticed that the DO in August 2018 was remarkably low (0.41 ppm) in both reservoirs, which could be the reason for high zooplankton diversity during the peak DO level periods in both reservoirs. Generally, tropical lakes depict negative correlation between DO and WT, which may ultimately decline the lake biodiversity including zooplankton diversity (Parmesan et al., 2022).
The low abundance of Cladocera and a greater abundance of Copepods, especially nauplii, were a clue for greater predation pressure (El-Bassat, 2008). A greater abundance of copepod nauplii during the wet season could be due to their parthenogenic reproduction strategy. Because of their minute body size, they can avoid the predators (Waya et al., 2017). The same phenomena may exist in TR as well. Although there were optimal conditions for zooplankton community, the total zooplankton abundance was decreased during the February 2019.
Temperature plays a major role in variations of zooplankton community structure. Karpowicz et al. (2020) revealed that, as a result of increasing AT, zooplankton cellular destructions can be occurred due to the exposure of upper surface water column to UV radiation. Larger Copepoda species are highly affected by this phenomenon and will lead to high mortality rates. Therefore, the Copepoda abundance may getting reduced further in both types of reservoirs with increasing AT. Current study revealed a significant positive correlation between AT and WT (r = 0.986; p = 0.001) in TR. Hence, it can suggest that the increasing AT leads to increased WT in TR and it was directly influenced on Copepoda abundance in this seasonal TR to record a significant (p = 0.015) negative correlation (r = − 0.912) with WT.
Though the critical minimum level for DO in a lake is about 4.5 ppm, Crustaceans, viz. Copepoda, can tolerate DO between 1 and 2 ppm with lethal DO 0.3–1.0 ppm (Karpowicz et al., 2020). However, the least value obtained for the DO in both reservoirs was 0.41 ppm, which is not lethal to Copepoda community. Thus, this may attribute with general morphological adaptations of Copepoda such as long and strong appendages to avoid predators to dominant even in low DO concentrations. Supportively, the increasing trend in zooplankton abundance with increasing DO also reported by Manickam et al. (2018) at Tamil Nadu, India.
Nevertheless, during dry season especially in August (2018), dissolved oxygen depletion can be considered as a risk and may lethal to survival of other Rotifers and Caldocerans, which could be a reason for vanishing of Rotifers such as Brachionus angularis, Brachionus budapestinensis, Keratella valga, Keratella cochlearis, Testudinella patina, Lecane bulla, Lecane closterocera, Lecane leontina and Cladocera such as Ceriodaphnia reticulata in both reservoirs.
Additionally, utilization of oxygen in the waterbody for respiration and organic matter decomposition ultimately can cause the decline in DO in the reservoirs (Manickam et al., 2018). Similarly, our present study also reflected as a negative correlation of DO with WT in both reservoirs (r = − 0.619 in VR; r = − 0.730 in TR).
Increasing \({\text{PO}}_{{4}}^{3 - }\) concentration leads to decline in zooplankton abundance in perennial VR. The studies done by Sangakkara and Wijeyaratne (2015); Haberman and Haldna (2014), for shallow lakes in Estonia and for Sri Lankan low country intermediate zone reservoirs also showed the similar results.
As per the USEPA (2000) standards, both reservoirs have a threat to become eutrophic water bodies in near future since they exceed the standard phosphate concentrations (> 0.024 ppm). Moreover, eutrophication is a phenomenon that can deplete the oxygen in a reservoir (Karpowicz et al., 2020). This can be observed in the current research results since nutrient concentrations (\({\text{NO}}_{{3}}^{ - }\) and \({\text{PO}}_{{4}}^{3 - }\)) have negative correlation (r = − 0.574, r = − 0.446) with DO in VR and (r = − 0.509, r = − 0.504) in TR, respectively.
Moreover, Sharma and Saini (2016) revealed that the presence of Filinia longiseta is the indicator of industrial effluent impact, and this species also observed in VR that indicates the risk of industrial effluent on VR. Additionally, Vavuniya reservoir is vulnerable to eutrophication that was reflected by the presences of Keratella cochlearis, which is an indicator of eutrophic freshwater bodies.
In addition, nitrate concentration was inversely associated with Copepoda and Rotifera abundance. And \({\text{PO}}_{{4}}^{3 - }\) concentration gives a negative effect on Cladocera community (Jeppesen et al., 2011). This might be the main reason that total zooplankton abundance also shows an inverse relationship with \({\text{NO}}_{{3}}^{ - }\) concentration in our study.
Water level, a crucial hydro-climatic factor, exhibited a noticeable variation between perennial Vavuniya reservoir and seasonal Thandikulam reservoir. Wang et al. (2020) revealed that both Cladocera diversity and abundance are significantly decreasing with increasing water levels. However, increasing WL during December 2018 in VR and January 2019 in TR can reduce the aquatic macrophyte and littoral vegetation, which are very important factors for the growth of Cladocera community. This may lead to loss of DO in freshwater ecosystem.
Supportively, the study also revealed that there was a significantly (p = 0.001) negative correlation (r = − 0.986) of WL with AT. Therefore, it can be suggested that increasing WT through increasing AT led to vaporization of water may attribute to lowering the WL only in the seasonal TR.
Conclusions
This study revealed that zooplankton community structure is highly depended on environmental factors, viz. \({\text{NO}}_{{3}}^{ - }\), \({\text{PO}}_{{4}}^{3 - } ,\) DO, atmospheric temperature and water level in both seasonal Thandikulam and perennial Vavuniya reservoir. Although these reservoirs are located at proximity, sensitivity and responses are seeming to be varied considerably between seasonal and perennial reservoirs.
Indeed, zooplankton community structure is highly sensitive to the nutrient loading in both seasonal and perennial reservoirs. Noticeably, zooplankton diversity and abundance in perennial VR are dependent on \({\text{NO}}_{3}^{-}\) concentration. Further, based on the nutrient loading observations, both reservoirs have a risk to become as a eutrophic water body in near future, which should be taken to consideration. As Filinia longiseta is ample evidence for industrial effluent in VR therefore, rice mill effluent should be monitored and controlled in ordered to conserve this reservoir. In concern of TR, the excess floating vegetation cover created by Eichhornia sp. should be removed to avoid autochthonous carbon and nutrient release into this water body due to the decaying process.
Seasonal reservoirs respond as reduction in zooplankton abundance with respect to decline in water level due to increment in WT and high demand for agricultural activities, which were directly correlated with increasing AT. Therefore, continuous monitoring of zooplankton communities under extreme drought events is necessary under prevailing climate change.
Since the statistical analysis results are aligned with the field observations, the current research is a motivation to use zooplankton community as a bio-sensor to assess environmental alterations in freshwater bodies in local and global context. The use of zooplankton community structure variations to assess the health of existing reservoirs in a cost-effective way is highly recommended.
Availability of data and materials
The data sets generated and /or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- VR:
-
Vavuniya reservoir
- TR:
-
Thandikulam reservoir
- RF:
-
Rainfall
- WL:
-
Water level
- AT:
-
Atmospheric temperature
- DO:
-
Dissolved oxygen
- WT:
-
Water temperature
References
Adrian, R., O’Reilly, M. O., Zagarese, H., Baines, S. B., Hessen, D. O., Keller, W., Livingstone, D. M., Sommaruga, R., Straile, D., Donk, E. V., Weyhenmeyer, G. A., & Winder, M. (2009). Lakes as sentinels of climate change. Limnology and Oceanography, 54(2), 2283–2297.
Aeriyanie, A. R., Sinang, S. C., Nayan, N., & Poh, K. B. (2021). Role of internal and external nutrients loading in regulating in-lake nutrient concentrations in a Eutrophic shallow lake. The International Journal by the Thai Society of Higher Education Institutes on Environment (environment Asia), 14(1), 63–68.
Banerjee, A., Chakrabarty, M., Rakshit, N., Bhowmick, A. R., & Ray, S. (2019). Environmental factors as indicators of dissolved oxygen concentration and zooplankton abundance: Deep learning versus traditional regression approach. Ecological Indicators, 100, 99–117. https://doi.org/10.1016/j.ecolind.2018.09.051
Bockwoldt, K. A., Nodine, E. R., Mihuc, T. B., Shambaugh, A. D., & Stockwell, J. D. (2017). Reduced phytoplankton and zooplankton diversity associated with increased cyanobacteria in Lake Champlain, USA. Journal of Contemporary Water Research and Education, 160(1), 100–118.
Eaton, A. D., Clesceri, L. S., Rice, E. W., & Greenberg, A. E. (Eds.). (2005). Standard methods: For the examination of water and wastewater (21st ed.). American Public Health Association.
El-Bassat, R. A. (2008). Composition and abundance of the zooplankton community in the Bitter Lakes, Egypt, in relation to environmental factors. African Journal of Aquatic Science, 33(3), 233–240.
Eriyagama, N., Smakhtin, V., Chandrapala, L. & Fernando, K. (2010). Impacts of climate change on water resources and agriculture in Sri Lanka: a review and preliminary vulnerability map**. Colombo, Sri Lanka: International Water Management Institute. 51. (IWMI Research Report 135), https://doi.org/10.3910/2010.211.
Haberman, J., & Haldna, M. (2014). Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Võrtsjärv. Journal of Limnology, 72(2), 61–71.
Impact of climate change on culture-based fisheries in seasonal reservoirs, Sri Lanka. (2012). Network of aquaculture centers in Asia-Pacific. https://enaca.org/?id=435
Jeppesen, E., Nõges, P., Davidson, T. A., Haberman, J., Nõges, T., Blank, K., & Johansson, L. S. (2011). Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia, 676(1), 279–297.
Jun, X., & Bantin, A.B. (2017). The impact of hydro climatic variability on water resources of Lake Chad Hydro Graphic Basin. Hydrology: Current Research, 8(3).
Karpowicz, M., Ejsmont-Karabin, J., Kozlowska, J., Feniova, I., & Dzialowski, A. R. (2020). Zooplankton community responses to oxygen stress. Water, 12(3), 706.
Manickam, N., Bhavan, P. S., Santhanam, P., Bhuvaneswari, R., Muralisankar, T., Srinivasan, V., Asaikkutti, A., Rajkumar, G., Udayasuriyan, R., & Karthik, M. (2018). Impact of seasonal changes in zooplankton biodiversity in Ukkadam Lake, Coimbatore, Tamil Nadu, India, and potential future implications of climate change. The Journal of Basic and Applied Zoology, 79, 15.
Mekong River Commission (2015). Identification Handbook of freshwater Zooplankton of the Mekong River and its tributaries. (MRC Technical Paper No.45). https://www.mrcmekong.org/assets/Publications/tech-No45-handbook-freshwater.pdf.
Parmesan, C., Morecroft, M.D., Trisurat, Y., Adrian, R., Anshari, G.Z., Arneth, A., Gao, Q., Gonzalez, P., Harris, R., Price, J., Stevens, N. & Talukdarr. (2022). Terrestrial and freshwater ecosystems and their services. In Climate change 2022: Impacts, adaptation and vulnerability, Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) Cambridge, UK: Cambridge University Press, 197–377, https://doi.org/10.1017/9781009325844.004.
Climate Change Secretariat; Ministry of Mahaweli Development and Environment. (2016). National Adaptation Plan for Climate Change Impacts in Sri Lanka (ISBN: 978-955-0033-98-0).
Riato, L., Ginkel, C. V., & Taylor, J. C. (2014). Zooplankton and diatoms of temporary and permanent freshwater pans in the Mpumalanga Highveld region, South Africa. African Zoology, 49(1), 113–127.
Sangakkara, S. M. A. I., & Wijeyaratne, M. J. S. (2015). Community structure of zooplankton and trophic status of some inland reservoirs in the low country intermediate zone of Sri Lanka. Sri Lanka Journal of Aquatic Sciences, 20(2), 59–74.
Shannon, C. E., & Weaver, W. (1949). The mathematical theory of communications (p. 117). University of Lllinois.
Sharma, K. K., & Saini, M. (2016). Impact of industrial effluents on diversity and population structure of rotifer fauna in river Basantar, Jammu (J&K, India). International Journal of Advanced Research, 4(5), 1458–1472.
Tian, W., Zhang, H., Zhang, J., Zhao, L., Miao, M., & Huang, H. (2017). Responses of zooplankton community to environmental factors and phytoplankton biomass in Lake Nansihu, China. Pakistan Journal of Zoology, 49(2), 493–504.
U S. Environmental Protection Agency (1983). Methods for chemical analysis of water and wastes. https://www.wbdg.org/FFC/EPA/EPACRIT/epa600_4_79_020.pdf
USEPA (2000). Nutrient Criteria—Technical Guidance Manual: Lakes and Reservoirs. First Edition. United States Environmental Protection Agency, Washington, DC, 232. https://www.epa.gov/sites/default/files/2018-10/documents/nutrient-criteria-manual-lakes-reservoirs.pdf
Vadadi-Fülöp, C., Sipkay, C., Mészáros, G., & Hufnagel, L. (2012). Climate change and freshwater zooplankton: What does it boil down to? Aquatic Ecology, 46(4), 501–519.
Wang, D., Kong, L., Wang, J., Huang, L., Liu, Y., & Chen, G. (2020). Spatiotemporal patterns of cladoceran community responses to water level variation in Haixi Lake, Southwest China. Inland Waters. https://doi.org/10.1080/20442041.2019.1672483
Waya, R. K., Limbu, S. M., Ngupula, G. W., Mwita, C. J., & Mgaya, Y. D. (2017). Temporal patterns in phytoplankton, zooplankton and fish composition, abundance and biomass in Shirati Bay, Lake Victoria, Tanzania. Lakes and Reservoirs: Research and Management, 22(1), 19–42.
Williamson, C. E., Saros, J. E., Vincent, W. F., & Smol, J. P. (2009). Lakes and reservoirs as sentinels, integrators, and regulators of Climate Change. Limnology and Oceanography, 54, 2273–2282.
Zannatul, F., & Muktadir, A. K. M. (2009). A review: Potentiality of zooplankton as bioindicator. American Journal of Applied Sciences, 6(10), 1815–1819.
Acknowledgements
The authors are gratefully acknowledged to Mr. G. Naveendrakumar, Senior Lecturer, Ms. H.K.N. Sanjeewani, Lecturer, Ms. K. Abarna, Lecturer; Department of Bio Science, Faculty of Applied Science, University of Vavuniya, Sri Lanka; for their support to attain a fruitful study. Also, the authors are thankful to the Laboratory staff, Department of Bio Science, Faculty of Applied Science, University of Vavuniya, Sri Lanka; for providing all necessary laboratory facilities. Furthermore, authors are acknowledged to the local fisherman community for their support to the sample collection in the field.
Funding
The study was carried with the existing facilities in the Department of Bio Science, Faculty of Applied Science, University of Vavuniya, Sri Lanka. No other form of funding involved in this study.
Author information
Authors and Affiliations
Contributions
CDK carried out the research under the guidance and the supervision of AESP. CDK drafted the manuscript and AESP reviewed before the initial submission of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We declare that this study does not require any ethical approval.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kottage, C.D., Patrick, A.E.S. Hydro-climatic consequences on zooplankton diversity and abundance in perennial Vavuniya reservoir and seasonal Thandikulam reservoir in Vavuniya, Sri Lanka. JoBAZ 84, 7 (2023). https://doi.org/10.1186/s41936-023-00328-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00328-8