Abstract
Background
Gemcitabine plus nab-paclitaxel (GnP) therapy is used for unresectable pancreatic ductal adenocarcinoma, but may cause interstitial lung disease (ILD) as a serious side effect. However, the risk factors for ILD in patients receiving GnP therapy are not well established. Here, we retrospectively investigated the incidence of GnP-induced ILD in pancreatic ductal adenocarcinoma patients, and the risk factors.
Methods
We investigated the patients’ background, laboratory data, previous treatment history, concomitant medications, number of doses of GnP, cumulative dosage and administration period, and occurrence of side effects.
Results
Of the 105 patients included in this study, ILD occurred in 10 (9.5%). Patients with ILD had a significantly higher frequency of concomitant treatment with Kampo medicines, especially gosha**kigan, which is considered to help prevent chemotherapy-induced peripheral neuropathy (CIPN) (odds ratio: 11.5, 95% confidence interval: 2.67–49.38). No significant differences were observed in other clinical characteristics. Notably, the severity of CIPN in patients who used gosha**kigan for prevention was not significantly different from that in patients who did not use gosha**kigan in this study.
Conclusions
These results suggest that administration of gosha**kigan to patients receiving GnP therapy for prevention of CIPN may need to be reconsidered.
Similar content being viewed by others
Introduction
Gemcitabine (GEM) plus nab-paclitaxel (nabPTX) (GnP) therapy has been widely used in Japan as chemotherapy for unresectable metastatic pancreatic ductal adenocarcinoma (PDAC) since it was approved in December 2014. In the Clinical Practice Guideline for Pancreatic Cancer 2019, GnP therapy is recommended as a first-line treatment along with FOLFIRINOX therapy (oxaliplatin + irinotecan + fluorouracil + levofolinate calcium) for locally advanced unresectable or metastatic PDAC [1].
However, interstitial lung disease (ILD) is a serious side effect of many anticancer agents, including GEM, nabPTX, and especially GnP [2], and is associated with high mortality. In a phase III international multicenter study in PDAC patients, the incidence of ILD was 1% in the GEM group and 4% in the nabPTX combination group [3]. According to the Japan Respiratory Society’s Guide for Diagnosis and Treatment of Drug-induced Pulmonary Disorders, non-specific risk factors for ILD include age 60 years or older, presence of existing lung disease (particularly interstitial pneumonia, pulmonary fibrosis), post-pulmonary surgery, deterioration of respiratory function, high-concentration oxygen administration, lung irradiation, multidrug therapy with anticancer drugs, heart disease, and renal disorders [4]. Specific risk factors for GEM-related ILD include elderly status, smoking, history of chemotherapy, advanced cancer stage, history of lung disease or lung cancer, and prior thoracic radiotherapy [5,6,7]. In addition, poor performance status has been reported as a risk factor for ILD in patients receiving several anticancer drugs [8, 9]. As for GnP therapy, Takeda et al. reported that ABO blood type B was an independent risk factor for ILD in PDAC patients [10]. Irie et al. found that the median age of GnP-treated patients with ILD was significantly higher than that of GnP-treated patients without ILD [11]. However, the risk factors for ILD in patients receiving GnP therapy have not yet been thoroughly investigated.
Therefore, we aimed to clarify the incidence of ILD in PDAC patients receiving GnP therapy, and to investigate the risk factors, in order to provide safer chemotherapy.
Materials & methods
Patients
The subjects were patients who underwent GnP therapy for locally advanced unresectable or metastatic PDAC at Kanazawa University Hospital from February 2015 to March 2019. Patients who received GnP only on day 1 were excluded. A standard 4-week course consisted of nab-PTX 125 mg/m2, GEM 1000 mg/m2 administered on days 1, 8 and 15, with a drug holiday on day 22.
Subjects for analysis
From the electronic medical records system, we collected data on pretreatment clinical characteristics, including age, gender, performance status (Eastern Cooperative Oncology Group), chemotherapy history, putative risk factors for ILD (history of lung disease, lung metastasis, lung surgery, thoracic radiotherapy, smoking, heart disease, and renal function), concomitant medications at the initiation of GnP treatment (non-steroidal anti-inflammatory agents (NSAIDs), proton pump inhibitors (PPIs), Kampo medicines, and pregabalin), administration period of concomitant medications, number of GnP doses, cumulative dosage and administration period, severity and time of onset of ILD, KL-6 level at the onset, clinical outcome, and the severity of chemotherapy-induced peripheral neuropathy (CIPN). Regarding concomitant drugs, we targeted drugs for which ILD is documented as a serious side effect, or that have been reported to cause ILD, and that were regularly used for 1 week or more, since onset of ILD is reported to occur at 1 to 6 weeks after the start of drug administration [4].
Evaluation method
The severity of ILD and CIPN was evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) ver.5.0. ILD was diagnosed based on a comprehensive evaluation of the patients’ clinical course, physical findings (cough, respiratory distress, fever, and decreased SpO2), X-ray findings, computed tomography findings (CT) and laboratory data (serum lactate dehydrogenase, C-reactive protein, KL-6, or surfactant proteins A or D). For differential diagnosis, absence of infection was confirmed by sputum and blood culture and serodiagnostic tests (1,3-β-d-glucan, cytomegalovirus antigenemia, or procalcitonin), or lack of response to antibiotics, in order to rule out infection or other pneumonia. Treatment of ILD was determined in consultation with a pulmonologist. The severity of CIPN was evaluated based on the medical records of physicians, nurses, and pharmacists at the time of discontinuation of GnP therapy or at the time of cutoff. If CIPN had developed before the initiation of GnP therapy, the onset of CIPN was defined as the time when CIPN worsened after the initiation of GnP therapy.
Statistical analysis
To examine risk factors, univariate analysis was performed for each factor using the Mann-Whitney U-test or Fisher’s exact test. P < 0.05 was considered statistically significant. The analysis was performed using SPSS ver. 24 statistical software (SPSS Co., Ltd., Tokyo).
Ethics
This study was conducted with the approval of the Kanazawa University Medical Ethics Review Committee in compliance with the “Ethical Guidelines for Medical Research for Humans” (Clinical Trial No. 2018–151).
Results
Patients’ characteristics
One hundred and seven patients who were treated with GnP therapy were registered. At the time of the data cutoff, 10 patients were still on treatment. Two patients were excluded because they were clinically diagnosed with progressive disease (PD) after a single dose, so the therapy was discontinued. Of the remaining 105 patients, 71 were males and 34 were females. The median age was 65 years. The number of patients receiving first-line treatment was 56, and the number receiving second-line or later treatment was 49. Prior treatment included modified FOLFIRINOX, S-1, GEM, and erlotinib. The median duration of treatment was 101 days and the median number of doses was 8 (Table 1).
Concomitant medications at the initiation of treatment
Concomitant medications at the initiation of GnP therapy were PPIs in 80 patients (76%), NSAIDs in 31 patients (30%), Kampo medicines in 30 patients (29%), and pregabalin in 8 patients (8%). Some patients were using multiple drugs. Among the Kampo medicines, gosha**kigan (GJG), prescribed to prevent exacerbation of CIPN, was the most common (23 people, 22%) (Table 2).
Frequency of occurrence of ILD
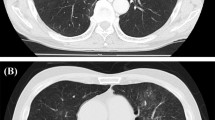
Of the 105 patients who received GnP, 10 (9.5%) developed ILD, with 5 Grade 1 (4.8%), 2 Grade 2 (1.9%), and 3 Grade 3 or higher (2.9%). The median period from initiation to onset was 79 days and the median number of cumulative doses was 8. After the ILD onset, GnP therapy was discontinued in all cases, and the symptom improved in response to steroid therapy in all patients with Grade 2 or more, and without treatment in the Grade 1 patients (Table 1, Fig. 1).
Risk factors for develo** ILD
To identify risk factors related to GnP-induced ILD, univariate analysis was performed using patient background factors and the presence or absence of ILD. GnP-induced ILD was significantly associated with the use of Kampo medicines, especially GJG (odds ratio: 11.5, 95% confidence interval (CI): 2.67–49.38). Other patient background factors were not significantly associated with the development of ILD (Table 3).
Demographics of patients with ILD
Demographics of the 10 patients who developed ILD are summarized in Table 4. Age was 60 years or older except for one patient, and there was no consistency in the number of GnP administrations and the date of onset. One of the 10 cases had a history of lung disease. In addition, 4 cases had a history of treatment with modified FOLFIRINOX as the first-line chemotherapy. Six cases showed increased KL-6 (> 500 U / mL) at the onset. Seven of 10 patients received GJG before the initiation of GnP treatment. There was no association between the duration of administration of GJG and the severity of ILD (P = 0.324).
Association between GJG preventive administration and CIPN severity
The incidence of CIPN among all cases and the association of CIPN severity with GJG preventive administration were evaluated. Among the patients receiving GnP, 72 (69%) developed CIPN, and the severity was Grade 1 in 41 (39%), Grade 2 in 24 (23%), and Grade 3 in 7 (7%). As the background of patients who be onset CIPN, there was no difference in age, sex, nabPTX cumulative dosage, the pretreatment history of oxaliplatin, and the history of CIPN at the initiation of GnP therapy between with and without GJG. Also, there was no difference in the use of pregabalin between with and without GJG. GJG administration appeared to have no protective effect against development of CIPN (Table 5).
Discussion
In this study, we investigated the incidence of ILD in PDAC patients receiving GnP therapy at our hospital. In the phase III international multicenter study of GnP therapy, the frequency of ILD was 4.0% (17/421) and the median period to develop ILD from initiation was 86 days [3]. Similarly, Takeda et al. reported that the incidence of ILD was 2.2% (20/910) in the PDAC patients receiving GnP therapy, and the median time to onset of ILD was 80 days [10]. On the other hand, Irie et al. observed a higher frequency of ILD in a small group of PDAC patients given GnP therapy (18.9%, 7/37), and reported that the severity of ILD was mild, except for one patient, who recovered with steroid therapy [11]. In our study, the incidence of ILD was 9.5% (10/105), and the median period to ILD onset was 79 days. Furthermore, ILD improved in all cases in response to treatment early after onset.
Next, we examined candidate risk factors for ILD and identified the administration of gosha**kigan used for CIPN prevention as a risk factor. GJG is composed of Achyranthis Radix, Rehmanniae Radix, Dioscoreae Rhizoma, Corni Fructus, Alismatis Rhizoma, Plantaginis Semen, Moutan Cortex, Poria, Processi Aconiti Radix and Cinnamomi Cortex, and is used to treat dysuria, pollakiuria, edema, low back pain, etc. It is often used in the treatment of CIPN based on its putative peripheral blood flow-promoting and antinociception effects [12, 13]. There have been three case reports suggesting that GJG caused ILD [14,15,16]. Those cases showed no increase in LDH or KL-6, and recovered in response to steroid treatment after discontinuing GJG. In our study, an increase in KL-6 was observed in 6/10 cases, but all recovered with steroid treatment after discontinuation of GJG, in line with the previous reports. We found no relationship between the period of GJG administration and the severity of ILD. The reported period from initiation of Kampo medicines to the appearance of ILD varied from several hours to 1 year [17,18,19], and it was unclear whether the appearance of ILD is dose-dependent. It was proposed that the induction of ILD by Kampo medicines is due to allergic reactions (mainly type III and type IV) [20], but the mechanism remains to be fully established. There has been no previous report on the onset of ILD caused by the combination of anticancer drugs and Kampo medicines. However, the number of cases of ILD was small in the present study, so further studies are needed to clarify the situation. Interestingly, we found no significant correlation between ILD onset and previously reported risk factors [4,5,6,7,8,9] in this study, but this may be due to the limited number of cases and the small numbers of comorbidities such as lung cancer and lung disease and poor performance status.
CIPN is a painful side effect for patients, and nabPTX contained in GnP can induce CIPN. Cryotherapy and the use of frozen gloves have been reported as preventive measures for CIPN [21, 22]. Prophylactic administration of GJG has also been suggested, though its effectiveness is unclear [23,24,25,26]. In this study, we found no preventive effect of GJG against CIPN. Furthermore, meta-analysis has indicated that GJG does not reduce the severity of CIPN in patients with colorectal cancer and breast cancer [27, 28]. In addition, the CIPN Management Guide of the Cancer Support Society states that administration of GJG is not recommended for the prevention of CIPN symptoms caused by oxaliplatin [29], although taxane drugs including nabPTX are not mentioned. Our results suggest that administration of GJG to prevent CIPN may not be desirable.
Limitations of this study include the small number of cases, reflecting the incidence rate of ILD, and therefore we could not perform multivariate analysis. In addition, unified regular respiratory symptom monitoring could not be performed due to the retrospective nature of the study. According to the Abraxane® Proper Use Guidelines (Taiho Yakuhin Kogyo Co., Ltd., December 2019 Revised), regular interviews with patients about initial symptoms such as fever, cough, shortness of breath, and dyspnea, auscultation, inspections of chest X-rays and CT, and clinical laboratory tests such as KL-6 values are recommended for early detection of ILD during GnP therapy. It will be important to develop a unified evaluation system for early detection of ILD in the hospital. Prospective trials based on the objective evaluation of ILD are also desirable. Also, we cannot rule out the possibility of that ILD was induced by GJG in some patients. At present, the frequency of GJG-induced ILD is unknown and there are only three reports of GJG-induced ILD to date. Finally, we could not confirm patients’ compliance with the prescribed GJG treatment during outpatient visits.
In conclusion, the incidence of ILD in PDAC patients treated with GnP therapy at our hospital was 9.5%, and coadministration of GJG appeared to be a risk factor for ILD. In addition, we found that preventive administration of GJG does not reduce the severity of CIPN. There is still no clear evidence that GJG can prevent CIPN, so GJG administration may need to be reconsidered, and in any event respiratory symptoms should be carefully monitored.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Committee JPCSPCPGR. Pancreatic cancer clinical practice guidelines 2019 edition. Tokyo: Kanahara Publishing; 2019. p. 223-239
Ogawa Y, Suzuki E, Mikata R, Yasui S, Abe M, Iino Y, et al. Five cases of interstitial pneumonitis due to gemcitabine and nab-paclitaxel combination treatment in pancreatic cancer patients. Pancreas. 2018;47(7):e42–3. https://doi.org/10.1097/MPA.0000000000001088.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. https://doi.org/10.1056/NEJMoa1304369.
Japan Respiratory Society. Guide for diagnosis and treatment of drug-induced pulmonary disorders. 2nd ed. Tokyo: Japan Respiratory Society; 2018. p. 3–14.
Hamada T, Yasunaga H, Nakai Y, Isayama H, Matsui H, Fushimi K, et al. Interstitial lung disease associated with gemcitabine: a Japanese retrospective cohort study. Respirology. 2016;21(2):338–43. https://doi.org/10.1111/resp.12665.
Furuse J, Gemma A, Ichikawa W, Okusaka T, Seki A, Ishii T. Postmarketing surveillance study of erlotinib plus gemcitabine for pancreatic cancer in Japan: POLARIS final analysis. Jpn J Clin Oncol. 2017;47(9):832–9. https://doi.org/10.1093/jjco/hyx075.
Umemura S, Yamase H, Suwaki T, Katoh T, Yano T, Shiote Y, et al. Interstitial lung disease associated with gemcitabine treatment in patients with non-small-cell lung cancer and pancreatic cancer. J Cancer Res Clin Oncol. 2011;137(10):1469–75. https://doi.org/10.1007/s00432-011-1013-1.
Hotta K, Kiura K, Takigawa N, Yoshioka H, Harita S, Kuyama S, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: the Okayama lung Cancer study group experience. J Thorac Oncol. 2010;5(2):179–84. https://doi.org/10.1097/JTO.0b013e3181ca12e0.
Gemma A, Kusumoto M, Kurihara Y, Masuda N, Banno S, Endo Y, et al. Interstitial lung disease onset and its risk factors in Japanese patients with ALK-positive NSCLC after treatment with crizotinib. J Thorac Oncol. 2019;14(4):672–82. https://doi.org/10.1016/j.jtho.2018.11.022.
Takeda T, Sasaki T, Fukuda K, Mie T, Furukawa T, Yamada Y, et al. Risk factors for gemcitabine plus nab-paclitaxel-induced interstitial lung disease in pancreatic cancer patients. Int J Clin Oncol. 2021;26(3):543–51. https://doi.org/10.1007/s10147-020-01827-2.
Irie H, Suzuki R, Takagi T, Sugimoto M, Konno N, Sato Y, et al. Interstitial lung disease in advanced pancreatic ductal adenocarcinoma patients treated with gemcitabine and nab-paclitaxel combination therapy: a retrospective analysis. Cancer Chemother Pharmacol. 2020;85(3):517–23. https://doi.org/10.1007/s00280-019-03983-3.
Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Antinociceptive effect of gosha-**ki-Gan, a Kampo medicine, in streptozotocin-induced diabetic mice. Jpn J Pharmacol. 1999;79(2):169–75. https://doi.org/10.1254/jjp.79.169.
Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Antinociceptive mechanism of gosha-**ki-Gan in streptozotocin-induced diabetic animals: role of nitric oxide in the periphery. Jpn J Pharmacol. 1999;79(3):387–91. https://doi.org/10.1254/jjp.79.387.
Katayama H, Hamada H, Yokoyama A, Kadowaki T, Ito R, Higaki J. A case of interstitial pneumonia caused by gosha-**ki-Gan. Jpn J Geriat. 2004;41(6):675–8. https://doi.org/10.3143/geriatrics.41.675.
Matsushima H, Takayanagi N, Tokunaga D, Maeno Y, Miyaoka K, Sato N, et al. A case of gosha-**ki-Gan induced interstitial pneumonitis and the review of the literature. Nihon Kyobu Rinsho. 2003;62:363–8.
Okamura K, Sakamoto H, Takiguchi J, Hashimoto Y, Inoue N, Onishi K. A case of gosha-**ki-Gan induced interstitial pneumonitis. Nihon Kyobu Rinsho. 2012;71:172–7.
Nakagawa A, Yamaguchi T, Takao K, Amano Y. Five cases of drug-induced pneumonitis due to Sho-saiko-to or interferon-alpha or both. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33(12):1361–6.
Sato A, Toyosima M, Kondo A, Ota K, Sato H, Osumi A. Pneumonitis induced by the herbal medicine Sho-saiko-to in Japan. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35(4):391–5.
Hatakeyama S, Tachibana A, Morita M, Suzuki K, Okano H. Five cases of pneumonitis induced by sho-saiko-to. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35(5):505–10.
Honma S, Nakada K. Yakuzaiseihaien. Igakutoyakugaku. 1996;35:29–37.
Hanai A, Ishiguro H, Sozu T, Tsuda M, Yano I, Nakagawa T, et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: prospective self-controlled trial. J Natl Cancer Inst. 2018;110(2):141–8. https://doi.org/10.1093/jnci/djx178.
Sato J, Mori M, Nihei S, Kumagai M, Takeuchi S, Kashiwaba M, et al. The effectiveness of regional cooling for paclitaxel-induced peripheral neuropathy. J Pharm Health Care Sci. 2016;2(1):33. https://doi.org/10.1186/s40780-016-0067-2.
Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, et al. Preventive effect of Gosha**kigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015;20(4):767–75. https://doi.org/10.1007/s10147-015-0784-9.
Kono T, Hata T, Morita S, Munemoto Y, Matsui T, Kojima H, et al. Gosha**kigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of gosha**kigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol. 2013;72(6):1283–90. https://doi.org/10.1007/s00280-013-2306-7.
Nishioka M, Shimada M, Kurita N, Iwata T, Morimoto S, Yoshikawa K, et al. The Kampo medicine, Gosha**kigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol. 2011;16(4):322–7. https://doi.org/10.1007/s10147-010-0183-1.
Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, et al. Objective evaluation of the alleviating effects of Gosha**kigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med. 2012;3(1):60–5. https://doi.org/10.3892/etm.2011.375.
Hoshino N, Ganeko R, Hida K, Sakai Y. Gosha**kigan for reducing chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Int J Clin Oncol. 2018;23(3):434–42. https://doi.org/10.1007/s10147-017-1229-4.
Kuriyama A, Endo K. Gosha**kigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer. 2018;26(4):1051–9. https://doi.org/10.1007/s00520-017-4028-6.
Japan Cancer Supportive Society. Guide to Management of Peripheral Neuropathy Associated with Cancer Pharmacotherapy, vol. 2017. Tokyo: Kanahara Publishing; 2017 Edition. p. 35–47.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RU conceived the study, designed the protocol, carried out the study, and drafted the manuscript. RU collected data from clinical records. NY, YH, KY, KO, TT, TS and YS coordinated the study and helped draft the manuscript. RU wrote the paper. All authors revised the manuscript for intellectual content and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted with the approval of the Kanazawa University Medical Ethics Review Committee in compliance with the “Ethical Guidelines for Medical Research for Humans” (Clinical Trial No. 2018–151).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ueda, R., Yamamoto, N., Hori, Y. et al. Risk factors for interstitial lung disease induced by gemcitabine plus albumin-bound paclitaxel therapy in pancreatic ductal adenocarcinoma patients. J Pharm Health Care Sci 8, 5 (2022). https://doi.org/10.1186/s40780-021-00236-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-021-00236-5