Abstract
Background
Drought stress is currently the primary abiotic stress factor for crop loss worldwide. Although drought stress reduces the crop yield significantly, species and genotypes differ in their stress response; some tolerate the stress effect while others not. In several systems, it has been shown that, some of the beneficial soil microbes ameliorate the stress effect and thereby, minimizing yield losses under stress conditions. Realizing the importance of beneficial soil microbes, a field experiment was conducted to study the effect of selected microbial inoculants namely, N-fixing bacteria, Bradyrhizobium liaoningense and P-supplying arbuscular mycorrhizal fungus, Ambispora leptoticha on growth and performance of a drought susceptible and high yielding soybean cultivar, MAUS 2 under drought condition.
Results
Drought stress imposed during flowering and pod filling stages showed that, dual inoculation consisting of B. liaoningense and A. leptoticha improved the physiological and biometric characteristics including nutrient uptake and yield under drought conditions. Inoculated plants showed an increased number of pods and pod weight per plant by 19% and 34% respectively, while the number of seeds and seed weight per plant increased by 17% and 32% respectively over un-inoculated plants under drought stress condition. Further, the inoculated plants showed higher chlorophyll and osmolyte content, higher detoxifying enzyme activity, and higher cell viability because of less membrane damage compared to un-inoculated plants under stress condition. In addition, they also showed higher water use efficiency coupled with more nutrients accumulation besides exhibiting higher load of beneficial microbes.
Conclusion
Dual inoculation of soybean plants with beneficial microbes would alleviate the drought stress effects, thereby allowing normal plants’ growth under stress condition. The study therefore, infers that AM fungal and rhizobia inoculation seems to be necessary when soybean is to be cultivated under drought or water limiting conditions.
Similar content being viewed by others
Background
The world's population is estimated to reach 10 billion by 2050 demanding additional 50% increase in food requirement [1]. The increased demand for food presents a significant challenge for the global food system, including increasing agricultural productivity and minimizing yield losses in major food crops. One of the factors posing a primary challenge to world’s food productivity is abiotic stress and more specifically, the drought stress which causes substantial yield loss in many food crops globally [2, 3]. Soybean [Glycine max (L.) Merrill], one of the world’s fastest growing protein-rich oilseed crops also suffers from drought stress causing a significant yield loss due to unprecedented rainfall and poor management. In India, the productivity is only about 25% of its potentiality as it is mainly grown as a rain-fed crop experiencing erratic, uneven and inadequate rainfall leading to a low productivity compared to other countries [4]. Besides water scarcity, nutrient deficiency and low nutrient use efficiency of the crop also causes low productivity of soybean [5] which is more sensitive to water deficits during the flowering, pod set and pod filling stages [6]. Therefore, in this scenario of unprecedented rainfall, there is a need to develop suitable cultivars tolerant to moisture stress or develop strategies to improve the stress tolerance of the existing cultivars through possible interventions [7, 8].
Beneficial soil microbes have been shown to offer several benefits including conferring drought and other abiotic stress tolerance to the host plants [9]. Microbial interactions with the plants are an integral part of the living ecosystem. They are natural partners modulating local and systemic mechanisms in plants offering defence under different developmental stages of a plant at molecular, physiological and biochemical level under various external stress conditions [10]. Symbiotic association of plants with certain beneficial microorganisms can enhance growth in host plants by mitigating the stress effects and providing profound benefits to the crop plants by enhancing the plant’s growth rate, stimulating the production of phyto-hormones, siderophores, etc. along with up-regulating the expression of dehydration response and antioxidant genes during abiotic stresses [9, 10].
Arbuscular mycorrhizal (AM) fungi are a type of beneficial fungi that form a symbiotic relationship with the roots of most vascular plants. They colonize the host roots and facilitate the plants to obtain nutrients and water from the soil and in-turn, the host plant provides the fungi with the required carbohydrates. Many studies have demonstrated that, AM fungal association helps to maintain bio-geochemical cycling even under drought conditions which play a critical role in ecosystem resilience [11]. Another group of symbiotic bacteria i.e. rhizobia accounts for 97% of the N fixation of the total plant N requirement [12, 13]. AM fungi when co-inoculated with bradyrhizobia may directly and preferentially stimulate rhizobial nodule function and have a more substantial impact on enhancing drought tolerance compared to rhizobia alone since AM fungi improve the plant’s growth through enhanced water and P uptake from soil, osmotic adjustment in roots, high leaf water potential, and reduced oxidative damage to lipids [14,15,16]. Although a large amount of information is available regarding the beneficial effects of microbes, the knowledge regarding the ‘on-field response’ of plants to simultaneous exposure to multiple stresses at different growth stages and the role of beneficial soil microbes in mitigating the stress effects is very scanty.
Literature survey suggests that, there needs to be more interaction studies, particularly under field conditions between rhizobia and AM fungi in soybean for alleviating the moisture stress effects [13, 17, 33]. In the present study, both Bradyrhizobium and AM fungal inoculation effectively regulated osmolytes in plants especially under moisture stress condition [34].
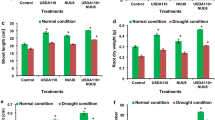
Influence of dual inoculation on a leaf osmolyte content and b proline content of leaves at flowering and pod filling stage of a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions. Dual inoculation: Ambispora leptoticha + Bradyrhizobium liaoningense; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress, Flowering stage: 1st stress period (35–60 DAS), Pod filling stage: 2nd stress period (85–100 DAS); Significant differences (p ≤ 0.05) relative to controls UI & UIS to their respective treatments I & IS are indicated by asterisk (*)
Proline, an amino acid and an important osmolyte is reported to increase several folds due to consequence of drought stress [35, 36]. Accordingly, in the dual inoculated plants, proline accumulation was significantly high compared to un-inoculated plants at both the stress periods (Fig. 3b). Further, the proline content was significantly high in stressed plants compared to irrigated plants. The increased proline levels in inoculated plants might have been triggered due to increased amino acid concentration, because of enhanced N supply by inoculated rhizobia present in root nodules [37]. Kohl et al. [38] have observed higher amounts of proline in soybean plants inoculated with Bradyrhizobium japonicum supporting our findings.
Drought stress induces the production of free radicals and reactive oxygen species (ROS) which affects cellular functioning leading to oxidative damage [39] and the ultimate death of plants [40]. However, in response to oxidative stress, plants produce ROS scavenging enzymes like catalase, guaiacol peroxidise (POX), superoxide dismutase (SOD), glutathione reductase and other enzymes which help to detoxify the ROS and protect the plants from oxidative damage. In the present field study, POX and SOD were analysed spectrophotometrically followed by native PAGE gel assay (analysed when plants were experiencing moisture stress during pod filling stage). This revealed a high content of POX and SOD enzymes in plants inoculated with microbial consortia compared to un-inoculated plants under both irrigated and stress conditions. Interestingly, the activity of the detoxifying enzymes was significantly more under stress conditions when compared to the control conditions with inoculated plants showing much higher activity (Fig. 4a). This was also evident from the SDA PAGE gel assay where, the POX and SOD iso-enzyme bands were thicker in samples of stressed plants compared to irrigated control plants (Fig. 4b). Within the stress treatment, samples of inoculated plants had visibly thicker bands compared to un-inoculated plants. Other reports also confirm the POX and SOD expression to be at higher levels in AM fungal colonized plants under moisture stress conditions [41]. Bressano et al. [42] have reported that, soybean plants exhibited increased resistance to oxidative stress caused by the herbicide, paraquat when subjected to dual inoculation of AM fungi and rhizobia. Our results therefore, suggest the importance of dual inoculation in reducing the oxidative stress damage to the plants under stress conditions. Various tests conducted in the present study showed that dual inoculation is necessary in soybean to reduce the effects of stress.
Influence of dual inoculation on a guaiacol peroxidase (POX) activity and b super oxide dismutase (SOD) activity along with their respective native PAGE assays of leaf samples analysed at pod filling stage of a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions. Dual inoculation: Ambispora leptoticha + Bradyrhizobium liaoningense; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress, Pod filling stage: 2nd stress period (85–100 DAS); Significant differences (p ≤ 0.05) relative to controls UI & UIS to their respective treatments I & IS are indicated by asterisk (*)
Influence of dual inoculation on growth and productivity of soybean: Several growth and yield parameters were measured in plants grown under different treatments. The specific leaf area (SLA), an indication of leaf thickness was low in dual inoculated plants compared to un-inoculated plants at both the stress periods (Fig. 5a). Furthermore, a lower specific leaf area indicates a thicker leaf and is often associated with greater tolerance to drought. This adaptation strategy can be attributed to the direct influence of AM fungal colonization, which is reported to supply water from deeper layers and nutrients from soil that results in the maintenance of a hydrated state in the plants and thereby, maintain better cellular functioning as compared to those without AM fungal colonization [43]. Total leaf area recorded at both the stress periods revealed that, dual inoculation significantly increased the leaf area in plants grown under both irrigated and stressed conditions with greater increase in irrigated control plants than in stressed plants (Fig. 5b; Additional file 3: Fig. S3). It is evident that, increased leaf area results in increased photosynthesis thereby increasing the plant yield. Increased leaf area under drought stress has been reported earlier in soybean when inoculated with a mixture of Bradyrhizobium japonicum, Rhizophagus intraradices and Funneliformis mosseae [44].
Influence of dual inoculation on a specific leaf area and b total leaf area analysed at flowering and pod filling stage of a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions. Dual inoculation: Ambispora leptoticha + Bradyrhizobium liaoningense; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress, Flowering stage: 1st stress period (35–60 DAS), Pod filling stage: 2nd stress period (85–100 DAS); Significant differences (p ≤ 0.05) relative to controls UI & UIS to their respective treatments I & IS are indicated by asterisk (*)
Plant growth parameters like plant height, stem diameter, biovolume index were significantly higher in dual inoculated plants under both irrigated and stressed conditions (Additional file 4: Table S1). AM fungal symbiosis along with bradyrhizobia in improving plant growth in soybean [44] and several other plants [45] is well documented. Such improvement in plant growth and establishment is attributed to the combination of enhanced water and nutrient uptake under moisture stress condition [17, 63] recorded improved nodule biomass of soybean with Funneliformis mosseae + Bradyrhizobium japonicum as efficient pair than Glomus deserticola + Bradyrhizobium japonicum. Nodule formation is highly affected in leguminous plants during the drought stress at physiological or molecular level which is found to be extremely sensitive but, co-inoculation with AM fungi improves nodule formation and N fixation which is well documented [64]. The importance of rhizosphere microorganisms [10, 65] and AM fungal influence on the rhizosphere population is also well documented [66, 67]. It is also reported that, rhizopshere microorganisms protect drought stressed plants through various strategies such as adjusting plant hormone levels, and producing osmolytes, antioxidants, and humectants [68]. Hence, it is important to analyse the rhizosphere population which depicts multiple roles of AM fungi in ameliorating moisture stress effects on plants. Qualitative analysis of all the microbial populations (bacteria, fungi, actinomycetes, N fixers, P solubilizers and Zn solubilizers) enumerated in the present study were significantly more in the rhizosphere soil of microbial consortia inoculated plants grown under drought stress conditions (Additional file 6: Table S3). A higher microbial population in the rhizosphere region is evident since the region contains more nutrients and metabolites due to root exudates which increase the microbial activity [69]. Quantitative analysis of dehydrogenase activity based on the reduction of 2,3,5-triphenyltetrazolium chloride (TTC) which analyzes the respiration of viable microorganisms was significantly higher in inoculated plants compared to un-inoculated plants (Additional file 6: Table S3). Many of these microbes are directly or indirectly involved in synergistic process with plant growth mechanisms and hence, it is very important for the plants also to have a healthy rhizosphere microbial population. Growth-promoting microbes in the rhizosphere stimulate the plant growth by synthesis of compounds facilitating the uptake of essential nutrient elements and production of plant growth regulators as well as through antagonistic activity towards plant pathogenic organisms and tolerate against abiotic stresses [10, 35].
Based on one-way and two-way AVOVA, the PLFA microbial biomarker groups were analysed and the results revealed that, the inoculated plants with microbial consortia (before or at stress) showed the presence of higher amounts of all the biomarkers over their respective un-inoculated control (Table 3). The PLFA and NLFA fractions were significantly enhanced due to microbial consortia involving the AM fungus A. leptoticha when compared to the un-inoculated plants. Irrespective of conditions (at stress or before stress), inoculation has significantly enhanced AM fungal biomarkers. The higher amounts of fungal to bacterial ratio and Gram-negative stress ratios in rhizosphere of inoculated plants indicate that, soils are less stressed due to the high biomass of AM fungi. In general, fungi are assumed to be less sensitive to changes in moisture and temperature than bacteria due to chitinous cell walls [70]. The principal component analysis (PCA) indicates a clear separation of treatments where, PC1 explains 43.1% of variations and PC2 explains13.2% of variations. The variation along the PC1 axis (43.1%) mainly explains Gram-negative stress ratio, AM fungi-PLFA and NLFA and actinomycetes variables. The big ellipse i.e. regular watered inoculated treatment contributed the highest in terms of the effect of treatments where the maximum contribution was made by AM fungal biomarkers, actinomycetes and Gram-negative stress ratio (Additional file 7: Fig. S4a). The small ellipses i.e. uninoculated plants at stress or regular watered had no biomarkers present (Additional file 7: Fig. S4a). When compared to the total variance, amongst all the variables, maximum contribution was made by AM fungal biomarkers (PLFA and NLFA both), actinomycetes followed by bacteria (Gram-negative and positive) (Additional file 7: Fig. S4b). It indicates that, PLFA biomarkers of AM fungi and actinomycetes act as major drivers in discerning the impact of stress in plants.
Influence of dual inoculation on plant nutrients status: Plant macro and micro-nutrients in general, decreased under moisture stressed conditions compared to regular watering. Microbial consortia inoculation significantly improved the nutrient uptake under stress (Table 4). The major nutrients such as N, P and K were significantly higher in inoculated plants which are important for plant's growth to withstand moisture stress. Mg was also higher in inoculated plants which is essential for chlorophyll formation. The role of AM fungi in P supply to plants and its importance in alleviating drought stress in plants is well documented [49]. Increased N is due to inoculated Bradyrhizobium through the N-fixation process [71]. Carbon absorption and distribution between plant shoot and root can be affected by an inadequate supply of P which negatively affects the functions and growth of nodules as P is needed for N fixation process for energy transformation in nodules to achieve maximum function [72, 73]. The inoculated AM fungus supplies the required amount of P, which plays a critical role in the nutrient exchange between the host plants and the fungus. Supporting the above results, Aliasgharzad et al. [50] have reported AM fungi with Bradyrhizobium japonicum increased plant N and K nutrients compared to un-inoculated soybean plants under pot culture trials.
The tripartite symbiosis in legumes, rhizobia and AM fungi is well documented and is one of the most important ecological mutualisms where the plants benefit with necessary N through rhizobial symbiosis and P through AM fungal symbiosis [12]. In turn, the rhizobia benefits with necessary P supply to both nodules and plants by AM fungi. The AM fungi in turn, benefits by photo-assimilates supply from the host plant [74]. The microbial consortia comprising of N-fixing bacteria Bradyrhizobium liaoningense + P-supplying AM fungi Ambispora leptiticha with abilities to supply necessary moisture and nutrients as and when required to the host plants conferred drought tolerance to the drought susceptible and high yielding soybean cultivar, MAUS 2 in the present study. Based on the various data recorded like physiological, biometric, yield, microbial and nutrient parameters, the inoculated microbial consortia in this study alleviated the moisture stress effect on the drought susceptible soybean cultivar, MAUS 2 and minimized the yield loss significantly. Symbiotic N fixation by inoculated rhizobia supplied major N requirement required by the plants at both flowering and pod filling stages and the AM fungi directly and preferentially stimulate the nodule function increasing N supply. Further, AM fungal symbiosis enhanced the plant growth through hyphal-mediated enhanced water uptake leading to enhanced osmotic adjustment in roots, maintaining high leaf water potential, reducing oxidative damage to lipids and supplying P from soil resulting in improved stress tolerance.
In general, a significant difference in physiological, growth and biometric parameters was observed between plants grown with and without dual inoculation. Further, plants grown under stress conditions with dual inoculation showed no significant difference with those grown under irrigated conditions with no inoculation suggesting the importance of dual inoculation in overcoming the stress effects in a drought susceptible soybean cultivar. Thus, the study under field condition revealed that, rhizobia and AM fungal inoculation is necessary for soybean in instances when the crop undergoes severe drought stress and alleviates the stress significantly at multiple growth and developmental stages.
Conclusion
The present study revealed that, dual inoculation with rhizobia B. liaoningense + AM fungus A. leptoticha has improved the physiological, biometric, yield, microbial and nutrient parameters of a drought susceptible and high-yielding soybean cultivar, MAUS 2. Under moisture stress conditions, dual inoculation increased the number of pods and pod weight per plant by 19% and 34% respectively; and the number of seeds and seed weight per plant by 17% and 32% respectively over un-inoculated plants. Thus, microbial consortia inoculation helped in nutrient mobilization, and water uptake and alleviated moisture stress in soybean cultivar, MAUS 2. Further, rhizobia plus AM fungal inoculation are necessary for soybean to alleviate drought stress at multiple growth and developmental stages.
Material and methods
Location, crop** and treatment details
The experiment was conducted during rabi (winter) season starting from October at the University of Agricultural Sciences, Bangalore (UASB) situated at an altitude of 920 m from mean sea level. The average maximum and minimum temperature that prevailed during the experimental period was 28 °C and 18 °C respectively, while the sunshine hours were 6.66 h and during the experimental period, a total rainfall of 110 mm was received. However, with the protective gear (protective cover/ canopy), moisture stress was imposed at the flowering and pod filling stages (incidentally, no rains received during the stress imposition period). The site where the experiment was conducted was used for raising finger millet crop prior to conduct of this experiment and was left fallow for three months. The crop** and treatment details are given under Additional file 8: Table S4.
Soil in the study area is classified as uniform, red sandy loam indicating consistent temperature regimes and the presence of kaolinite clay minerals that contribute to its fertility. Soil samples collected from this area was analysed for various physico-chemical properties and the data is presented below. The soil pH was 6.2 determined using digital pH meter, while, the electrical conductivity analysed with the help of the Conductivity Bridge measured 0.11 dS/m. Soil organic carbon and available N, P and K was 0.45%, 259.94; 28.40 and 207.61 kg/ha respectively which were estimated following the standard procedures [75].
Native AM fungal spore population was 20 spores/g of soil and the rhizobial population was not observed as evidenced from serial dilution plating method using YEM agar medium estimated following standard procedures [76]. The physiological and microbial parameters were analysed at UASB, Bangalore and at the Centre for Natural Biological Resources and Community Development (CNBRCD), Bangalore respectively.
Soybean variety
The experiment was performed with a high yielding and drought susceptible soybean cultivar, MAUS 2 [Germplasm Accession No. MAUS 2; UASB]. The cultivar was selected based on an earlier field experiment conducted to investigate the drought tolerance abilities along with various other varieties (unpublished data). The shortlisted cultivars were then screened with different AM fungi [76] followed by another drought adaptive trials with single and dual inoculation of selected rhizobia and AM fungus conducted at greenhouse conditions [77]. Later, a micro-plot experiment was conducted with drought susceptible cultivar, MAUS 2 and a drought tolerant cultivar, DSR 12 [Germplasm Accession No. HARDEE; ICAR-IISR, Indore] to study their stress response through inoculation with microbial consortia (rhizobia and AM fungi) [19].
Ambispora leptoticha inoculum
The AM fungus, Ambispora leptoticha being an obligate symbiont was multiplied in plastic pots using the substrate mix containing vermiculite, perlite and soilrite in the ratio of 3:1:1 v/v/v under glasshouse conditions and Rhodes grass (Chloris gayana Kunth) as the host. After 75 days of growth, shoots of Rhodes grass were severed and the substrate containing spores, hyphae and root bits (cut into about 1 cm pieces) was air dried and used as the inoculum. The infective propagule (IP) numbers of the AM fungus was estimated by the most probable number (MPN) method [78] and was found to be 1700 IP/g of inoculum.
Bradyrhizobium liaoningense inoculum
B. liaoningense sp. nov. (MTCC 10753/NCBI No. JF792426) was procured from Indian Council of Agricultural Research-Indian Institute of Soybean Research (ICAR-IISR), Indore, India. Bradyrhizobium liaoningense was used in this experiment as it was proven to be the best rhizobial species for improving growth of soybean [79]. The procured culture was sub-cultured on yeast extract mannitol (YEM) agar medium with Congo red and incubated at 28 °C for 5 days. Pure isolated single colonies were picked and inoculated to YEM broth and incubated at 28 °C for 5 days. Fully grown broth culture was cold centrifuged and the bacterial pellets collected was mixed in dilute phosphate buffer and used as inoculum. The rhizobial population of the inoculum mixed in phosphate buffer was estimated by serial dilution and plating method which had 1 × 109 CFU/ml of inoculum. The inoculum was added to 10% starch solution (1:1 v/v) and this inoculum mixture was mixed with soybean seeds to form a seed coat. The coated seeds were shade dried and sown. The population of B. liaoningense coated on the seeds was enumerated by serial dilution method and was found to be 1 × 106 CFU/seed.
Experimental setup
The study plot was prepared, brought to a fine tilth and farmyard manure (FYM) was applied and mixed thoroughly with soil. Using randomized block design, plots with four different treatments having 4 replications were made. Chemical fertilizers (procured from Zuari Agro Chemicals Ltd, Bangalore) i.e. nitrogen in the form of urea (46% N) @ 66.66 kg/ha, phosphorus in the form of SSP (16% P2O5) @ 500 kg/ha and potassium in the form of MOP (60% K2O) @ 63.33 kg/ha were applied in seeding rows.
Ambispora leptoticha was added @ 10 g/plant evenly in the furrows made in the plots according to the details of the treatments [76]. Control plots received only the substrate vermiculite, soilrite and perlite (3:1:1 v/v/v basis). B. liaoningense coated seeds of soybean cultivar, MAUS 2 were sown (3 seeds/ seeding point, later thinned to leave 1 seedling) in furrows. Control plots were sown with seeds coated with starch. Plots were irrigated after sowing. Later, protective irrigation was given once every 3 days. T1 and T2 plots were irrigated till harvest while, moisture stress was imposed on T3 and T4 treatments at 2 different developmental stages of the crop growth period. Irrigation was stopped and moisture stress was imposed at the early reproductive phase for 25 days when the plants start flowering from 35 to 60 days after sowing (DAS) and re-irrigated from 61 to 84 DAS to alleviate the stress and to allow the plants to recover from stress effects. Moisture stress was imposed again for 15 days from 85 to 100 DAS when the plants were at the pod filling stage. Later, these plots were irrigated till maturity and harvest. In a nutshell, the crop was imposed with moisture stress at two crucial developmental stages namely, flowering and pod filling stages to examine whether, the microbial consortia have any role in overcoming the stress effect and thus, imparting stress tolerance to the soybean plants.
All the physiological and biometric parameters described below were analysed at both the stress periods (35–60 DAS and 85–100 DAS). However, leaf osmotic concentration, peroxidase and superoxide dismutase enzyme activity were analysed during1st stress period between 35 and 60 DAS and the water use efficiency by carbon isotope discrimination (Δ13C) approach was analysed during 2nd stress period between 85 and 100 DAS. The parameters analysed following the standard methodologies as described by earlier workers [19, 77, 80, 81].
Measurement of physiological parameters
Soil physical parameters
Soil physical parameters viz. soil moisture, soil temperature and soil water potential were analysed during both the stress periods (35–60 and 85–100 DAS). The soil sample from a depth of 0–10 cm was collected from each plot and brought to laboratory for analysis. Soil moisture was estimated by gravimetric method using the formula.
Soil temperature (oC) and soil water potential (ψ) was measured using an Dew point potentiameter device (WP4 Dewpoint Potentiometer manufactured by Decagon Devices, Inc., USA).
Relative water content
Relative water content (RWC) was measured by analysing fresh weight (FW), turgid weight (TW) and dry weight (DW) of leaves. The following formula was used to determine RWC [80, 81].
Cell membrane damage
Cell membrane damage was analysed as outlined by Leopold et al. [82] by measuring the intensity of solutes leaked out of the leaf cells. The leaf samples of known weight were incubated in water for 3 h in a glass beaker and the leachate (L0) was measured at 273 nm in a spectrophotometer (Spectronic 20D + by Thermo Scientific, USA). Later, the leaves were boiled for 15 min at 100 °C and the leachate (L1) absorbance was recorded. The percent leakage was calculated as per the formula given below.
Cell viability
Cell viability was measured by 2,3,5-triphenyl tetrazolium chloride (TTC) reduction test [83]. The viability of the tissue is reflected as colour formation when TTC is reduced to red formazon in living and respiring tissues. The intensity of the colour formation was measured by recording the absorbance at 485 nm in spectrophotometer (Spectronic 20D + by Thermo Scientific, USA). The absorbance values indicate the direct reflection of leaf cell viability.
Leaf chlorophyll status (SPAD value, total chlorophyll content and chlorophyll stability index)
Using a portable SPAD (Soil Plant Analysis Development) meter (Minolta Corp., Ramsey), a non-destructive analysis of chlorophyll/ nitrogen status in the leaf was measured by clam** the SPAD meter onto the leaf at different positions as well as on different leaves. The mean of several SPAD Chlorophyll Meter Readings (SCMR) for each treatment was calculated and presented as unit less SCMR value. Using UV–Visible spectrophotometer (SpectraMax Plus 384 by Molecular Devices, USA), the total chlorophyll content (TCC) and chlorophyll stability index (CSI) were determined following the modified method of Hiscox and Israelstam [84]. The values obtained were then substituted in the below-mentioned equation to determine total chlorophyll content (TCC) and chlorophyll stability index (CSI).
Where, \(R\hspace{0.17em}=\hspace{0.17em}[(control-stressed)/control]\hspace{0.17em}\times \hspace{0.17em}100\)
(Note: A-Absorbance, V-Volume of acetone and DMSO solution)
Leaf osmolyte content
Leaf samples from 5 plants from all the plots were collected, wrapped in aluminium foil and frozen in liquid nitrogen. The samples were then thawed and centrifuged for 5 min at 12,000 rpm and the extracted sap was collected. The extracted sap was measured for leaf osmolyte content using VAPRO vapour pressure osmometer (WescorInc, Logan, USA).
Proline estimation
Proline increases proportionately under water stress faster than other amino acids in plants and is one of the important osmolytes accumulated under stress which makes the plants to absorb water even under low soil water status by kee** the root water potential lower than the soil. The analysis was carried out based on the procedure given by Bates [85].
Assessing the activity of ROS detoxifying enzymes
Stress generally induces the production of free radicals and ROS damaging the membrane system. In response to this, plants also have a defensive mechanism where through the production of detoxifying enzymes, they could control the damage caused by ROS and other toxic compounds. In this study, the defensive enzymes such as POX and SOD were analysed in the samples collected during 2nd stress period (85–100 DAS) coinciding with pod filling stage to examine whether or not the dual inoculation has any role in regulating the stress effects through the defensive mechanism.
Protein extraction: Plant leaf samples from all the treatments were collected and frozen using liquid nitrogen to prevent proteolytic activity before being homogenized using a pestle and mortar and the homogenate was suspended in extraction buffer [Phosphate buffer 0.1 M, pH 7.8, 1 mM PMSF (protease inhibitor) and 0.1% of polyvinylpyrollidon (PVP)] in an Eppendorf tube and kept on ice for 15 min. The crude protein extract was centrifuged at 14,000 rpm at 4 °C for 30 min in a cooling centrifuge. Later, the pellet was discarded and the soluble protein supernatant was used for further analysis. Protein concentration was determined by Lowry’s method [86] using bovine serum albumin (BSA) as standard.
Enzyme assays: POX enzyme activity in the protein extract was measured by the method proposed by Castillo et al. [87] with slight modification. Peroxidase activity was assayed as the increase in optical density due to the oxidation of guaiacol to tetra-guaiacol. Native PAGE was performed as per the method described by Davis [88] for peroxidase isoenzyme activity using 10% resolving gel and 5% stacking gel. Protein extract (25 μg) of all the treatments were loaded to the gel separately. Gel electrophoresis was run initially at 80 V and later, once protein entered the resolving gel, increased to 120 V. Electrophoresis was conducted at 40 °C for about 3 h. Later, the gel was stained for peroxidase isoenzymes.
SOD activity was measured by the method described by Dhindsa et al. [89] with slight modifications. SOD activity in the supernatant was assayed by its ability to inhibit photochemical reduction of nitroblue tetrazolium. Native PAGE was performed according to the method described by Davis [88] for superoxide dismutase isoenzyme activity using 5% stacking gel and 10% resolving gel. Protein extract (25 μg) from all the sample treatments were loaded to gel. Electrophoresis was performed initially at 60 V and after the protein entered the resolving gel, the voltage was increased to 120. The electrophoresis was conducted for 3 h at 40 °C. The gel was incubated in a staining solution containing 100% NBT (nitroblue tetrazolium chloride) (w/v), 0.2 M EDTA (Ethylenediaminetetraacetic acid) (w/v), 0.1 M sodium phosphate buffer (pH 7.5), commercial grade TEMED (Thermo Scientific Pierce Tetramethylethylenediamine) and 5% riboflavin (w/v) for 30 min until the bands appeared. The isoenzyme bands appeared as white/colourless in a dark blue background and the isoenzyme pattern was photographed.
Measurement of growth and yield attributes
Specific leaf area
Specific leaf area (SLA), an expression of leaf thickness was determined as per the formula given below [80, 81].
Total leaf area
Total leaf area (TLA) was recorded at two stress periods and at harvest by measuring the specific leaf area of 5 randomly selected leaves. WinDIAS 3 Image Analysis System [90] and electronic weighing balance were used to measure leaf area and the leaf dry weight respectively. Later, at harvest, all the leaves were separated and collected from the plant, oven dried and multiplied with SLA to get the total leaf area.
Plant height, stem diameter, biovolume index and days to 50% flowering
The plant growth parameters like plant height and stem diameter were determined before harvest. Plant height was recorded from the soil surface to the growing tip of the plant using a measuring scale and the stem diameter was measured just above the soil surface using digital Vernier Callipers. Biovolume index (BI) was calculated by the formula given by Hatchell et al. [91]. The plant growth parameters were also recorded during the stress period (35–60 DAS) and before harvest; however, only the final plant growth parameter readings recorded at harvest are presented. Plants were monitored regularly for the appearance of the first flower. The number of days taken from sowing to flowering was recorded.
Plant dry biomass and root volume
After harvest, plants’ shoot and root parts were separated from 20 sampled plants and collected in paper bags individually. The plant material was dried in a hot air oven (manufactured by Servewell Instruments Pvt. Ltd., Bangalore) at 60 °C to a constant weight. Later, the weight of shoot and root of each replication was weighed on a standard weighing balance. Total dry biomass of plant was calculated by summing up both shoot and root biomass. In order to determine the net plot biomass and yield, all the plants in the plot were uprooted, plant (shoot + root) and pods separated, and dried. The net plot biomass from each plot and yield was noted. Root volume per plant was determined by the water displacement method outlined by Harrington et al. [92]. The root samples separated from harvested plants were submerged in a graduated beaker containing known amount of water. The volume of water displaced when root is submerged in water was measured and expressed as cm3 per plant.
Yield and harvest index
Pods from 20 randomly selected plants were collected at maturity and individual plant pod yield was recorded. Later, the seeds were separated from the pod and the number of seeds/ plant and weight of seeds/ plant were recorded. All the plants grown in each plot were harvested to determine the net plot pod and seed weight. Harvest index (HI), a measure of reproductive efficiency was calculated by using the formula given by Donald and Hamblin [93].
Determination of WUE based on carbon isotope discrimination (Δ13C) technique
In nature, there exists two stable isotopes of carbon viz., 12C and 13C of which, major carbon share is 12C with 98.9% and rest 1.1% is 13C. Overall, 13C abundance relative to 12C in the plant is less common. The Δ13C in plant samples was determined using a sophisticated analytical instrument called Isotope Ratio Mass Spectrometer (IRMS). The plant sample is converted to CO2 by combustion and the isotopic composition is determined. Leaf samples collected to measure SLA were subjected to carbon isotope discrimination analysis [80, 81]. The leaf samples were dried and finely powdered with a metal beads and 1 mg of finely powdered sample was then taken in silver capsules and crimped and placed sequentially in the carousel of the auto-sampler. The samples were then dropped at precise times along with an injection of pure O2 into the oxidation reactor. Once the sample combustion takes place inside the instrument, the instrument records the C discrimination values (Δ13C values). The analysis to determine the carbon isotope discrimination using IRMS was done at a National Facility for Stable Isotope Studies in Biological Sciences installed at the Department of Crop Physiology, UAS, Bangalore.
Microbial parameters
Rhizobial and mycorrhizal parameters
Rhizobial nodule numbers were counted manually and the weight was recorded from 20 randomly selected plants under each treatment at harvest. The mycorrhizal parameters like per-cent root colonization was carried out as per the procedure proposed by Philips and Hayman [94] and spore numbers in the root zone soil was determined by wet sieving and decantation procedure as outlined by Gerdemann and Nicolson [95].
Enumeration of rhizosphere beneficial microflora
The rhizosphere samples were collected at harvest from 5 plants from all the replications under each treatment in a polythene cover and immediately brought to the laboratory and stored at − 20 °C for further analysis. The samples during analysis were pooled, and taken as representative composite samples under each treatment. The total bacterial, fungal, actinomycetes and N-fixers population were determined using spread plate technique using Soil Extract Agar, Martin’s Rose Bengal Agar, Kenknight and Munaier’s Agar, Combined Carbon Agar medium respectively [96].
Dehydrogenase activity in rhizosphere soil
Dehydrogenase activity in soil serves as an indicator of microbial oxidative activities and is a measure of total microbial activity. The activity was determined by the procedure given by Casida et al. [97] using 2, 3, 5-triphenyl tetrazolium chloride (TTC). The microbial activity is reflected as colour formation when TTC is reduced to red formazon and intensity of colour formation was measured by recording the absorbance at 485 nm in a spectrophotometer (Spectronic 20D + by Thermo Scientific, USA).
Phospholipid fatty acid extraction (PLFA) and quantification
PLFA analysis was carried out on column chromatography and lipids were analysed on gas chromatograph with flame ionization detector (GC-FID). PLFA biomarkers specific to microbial community including AM fungi were quantified. PLFA were analysed as per the method described by Buyer and Sasser [98] & Sharma and Buyer [99]. About 2 g of freeze-dried rhizosphere soil samples were used to extract the lipids by solid-phase extraction (SPE) followed by extraction of phospholipids by SPE. The chloroform fraction from the SPE was used for neutral lipid fatty acids (NLFA) analysis while, the 5:5:1 (chloroform: methanol: water) fraction was used for PLFA analysis. NLFA and PLFA fractions were converted to fatty acid methyl esters by transesterification and analysed by gas chromatography. The PLFAs were summed into biomarker categories as follows: gram-positive bacteria, Gram-negative bacteria, anaerobe, eukaryote, fungi, actinomycetes, AM fungi (PLFA), AM fungi (NLFA). FAME profiles of PLFA were identified using the MIDI PLFAD1 calibration mix and peak naming table (MIDI, Inc., DE, USA) and from NLFA run, only profile of 16:1ω5cis was used representing AM fungal biomass.
Macro- and micro-nutrients in plant tissue
The plant N, P and K concentration was estimated following the Micro-kjeldahl method, vanadomolybdate phosphoric yellow colour method and flame photometer method respectively [75]. The plant Ca and Mg concentrations were estimated by EDTA titration method [75]. The plant micro nutrients Zn, Fe, Cu, Mn and Bo were estimated by atomic absorption spectroscopy [100].
Statistical analysis
Raw data of each parameter were subjected to one-way analysis of variance (ANOVA) at a significance level of 5% and means were compared by Duncan’s multiple range test (DMRT) when F-values were significant using Costat statistical software (Costat/Cohort statistical software, CA, USA) and AgRes Statistical Software (Ver. 3.01) by Pascal Intl. Software Solutions and SAS Institute Inc. [101]. Microbial fatty biomarkers data were subjected to Principal Component Analysis (PCA) to determine the main contributing factors to the total variance across the stress and inoculation treatments. PCA was performed in R version 3.6.0 by using R packages, ‘factoextra’, ‘FactoMineR’, ‘devtools’ and ‘ggbiplot’ [98, 99].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
OECD-FAO. OECD-FAO agricultural outlook 2018–2027. Paris: OECD Publishing; 2018. https://doi.org/10.1787/agr_outlook-2018-en.
Kang SM, Khan AL, Waqs M, et al. Plant growth promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 2014;9:673–82.
Rosa L, Chiarelli DD, Rulli MC, Dell’AngeloD’Odorico JP. Global agricultural economic water scarcity. Sci Adv. 2020;6:eaaz6031. https://doi.org/10.1126/sciadvaaz6031.
Agarwal DK, Billore SD, Sharma AN, Dupare BU, Srivastava SK. Soybean: introduction, improvement, and utilization in India-problems and prospects. Agric Res. 2013;2:293–300.
Bagheri A, Sadeghipour O. Biochemical changes of common bean (Phaseolus vulgaris L.) to pretreatment with salicylic acid (SA) under water stress conditions. Int J Biosci. 2012;2:14–22.
Eck HV, Mathers AC, Musick JT. Plant water stress at various growth stages and growth and yield of soybeans. Field Crops Res. 1987;17(1):1–16.
Rockström J, Williams J, Daily G, Noble A, Matthews N, Gordon L, Wetterstrand H, DeClerck F, Shah M, Steduto P, et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio. 2017;46:4–17. https://doi.org/10.1007/s13280-016-0793-6.
Miralles-Wilhelm F. Nature-based solutions in agriculture: sustainable management and conservation of land, water and biodiversity. Virginia: Food & Agriculture Organization of the UN and The Nature Conservancy; 2021.
Koza NA, Adedayo AA, Babalola OO, Kappo AP. Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms. 2022;10:1528. https://doi.org/10.3390/microorganisms10081528.
Meena KK, Sorty AM, Bitla UM, et al. Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci. 2017;8:00172. https://doi.org/10.3389/fpls201700172.
Mohan JE, Cowden CC, Baas P, et al. Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol. 2014;10:3–19.
Antunes PM, Goss MJ. Communication in the tripartite symbiosis formed by arbuscular mycorrhizal fungi, rhizobia, and legume plants: a review. Agronomy. 2005;48:199.
Bharti A, Agnihotri R, Maheshwari HS, Prakash A, Sharma MP. Bradyrhizobia-mediated drought tolerance in soybean and mechanisms involved. In: Choudhary D, Kumar M, Prasad R, Kumar V, editors. In silico approach for sustainable agriculture. Singapore: Springer; 2018. p. 121–39.
Ruiz-Lozano JM, Collados C, Barea JM, Azcón R. Arbuscular mycorrhizal symbiosis can alleviate drought-induced nodule senescence in soybean plants. New Phytol. 2001;151:493–502.
Sharma MP, Jaisinghani K, Sharma SK, Bhatia VS. Effect of native soybean rhizobia and AM fungi in the improvement of nodulation, growth, soil enzymes and physiological status of soybean under microcosm conditions. Agric Res. 2012;1:346–51.
Amani MM, Javanmard A, Morshedloo MR, Aghaee A, Maggi F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercrop** with soybean. Sci Rep. 2021;11:15279.
Igiehon ON, Babalola OO. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr Microbiol. 2021;78:1615–27.
Sheteiwy MS, Ali DFI, **ong YC, et al. Physiological and biochemical responses of soybean plants inoculated with arbuscular mycorrhizal fungi and bradyrhizobium under drought stress. BMC Plant Biol. 2021;2:195.
Ashwin R, Bagyaraj DJ, Raju BM. Dual inoculation with rhizobia Bradyrhizobium liaoningense and arbuscular mycorrhizal fungus Ambispora leptoticha improves drought stress tolerance and productivity in soybean cultivars MAUS 2 and DSR 12 under microplot conditions. Biologia. 2022. https://doi.org/10.1007/s11756-022-01196-3.
Bárzana G, Aroca R, Paz JA, et al. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot. 2021;109(5):1009–17.
Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L. Aquaporins in plants. Physiol Rev. 2015;95:1321–58.
Aroca R, Bago A, Sutka M, Paz JA. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol Plant-Microbe Interact. 2009;22(9):1169–78.
Armada E, Azcón R, López-Castillo OM, Calvo-Polanco M, et al. Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded Mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Physiol Biochem. 2015;90:64–74.
Duc NH, Posta K. Mycorrhiza-induced alleviation of plant disease caused by Clavibacter michiganensis sub sp michiganensis and role of ethylene in mycorrhiza-induced resistance in tomato. Acta Biol Hung. 2018;69(2):170–81.
Calvo-Polanco M, Sánchez-Castro I, Cantos M, García JL, Azcón R, Ruiz-Lozano JM, et al. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant Cell Environ. 2016;39(11):2498–514.
Jia-Dong H, Tao D, Hui-Hui W, Zou YN, Wu QS, Kamil K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci Hortic. 2019;243:64–9.
Nisha K, Kuldeep Y, Ashok A. Application of AM fungi with Bradyrhizobium japonicum in improving growth, nutrient uptake and yield of Vigna radiata L under saline soil. J Stress Physiol Biochem. 2019;10(3):134–52.
Evelin H, Devi TS, Gupta S, Kapoor R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci. 2019;10:470. https://doi.org/10.3389/fpls2019.00470.
Evelin H, Giri B, Kapoor R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl stressed Trigonella foenum-graecum. Mycorrhiza. 2012;22:203–17.
Tairo VE, Ndakidemi AP. Bradyrhizobium japonicum inoculation and phosphorus supplementation on growth and chlorophyll accumulation in soybean (Glycine max L). Am J Plant Sci. 2013;4:2281.
Wu N, Li Z, Wu F, Tang M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci Rep. 2016;6:37663.
Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25(1):13–24.
Serraj R, Sinclair TR. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002;25(2):333–41.
Chitarra W, Maserti B, Gambino G, Guerrieri E, Balestrini R. Arbuscular mycorrhizal symbiosis-mediated tomato tolerance to drought. Plant Signal Behav. 2016;11(7):1009–23.
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–98.
Sánchez FJ, Manzanares M, de Andres EF, Tenorio JL, Ayerbe L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998;59(3):225–35.
Ramos MLG, Parsons R, Sprent JI. Differences in ureide and amino acid content of water stressed soybean inoculated with Bradyrhizobium japonicum and B. elkanii. Pesq Agropec Bras. 2005;40(5):453–8.
Kohl DH, Kennelly EJ, Zhu Y, Schubert KR, Shearer G. Proline accumulation, nitrogenase (C2H2 reducing) activity and activities of enzymes related to proline metabolism in drought-stressed soybean nodules. J Exp Bot. 1991;42(7):831–7.
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol. 2004;55:373–99.
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–67.
Wu QS, Zou YN, Abd-Allah EF. Mycorrhizal association and ROS in plants. In: Ahmad P, editor. Oxidative damage to plants. Cambridge: Academic Press; 2014. p. 453–75.
Bressano M, Curetti M, Giachero L, Gil SV, Cabello M, March G, Ducasse DA, Luna CM. Mycorrhizal fungi symbiosis as a strategy against oxidative stress in soybean plants. J Plant Physiol. 2010;167(18):1622–6.
Ruiz-Lozano JM, Azcón R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant. 1995;95(3):472–8.
Takács T, Cseresnyés I, Kovács R, Parádi I, et al. Symbiotic effectivity of dual and tripartite associations on soybean (Glycine max L. Merr) cultivars inoculated with Bradyrhizobium japonicum and AM fungi. Front Plant Sci. 2018;9:1631. https://doi.org/10.3389/fpls.2018.01631.
Bagyaraj DJ. Microbial biotechnology for sustainable agriculture, horticulture and forestry. New Delhi: New India Publishing Agency; 2011.
Shavrukov Y, Kurishbayev A, Jatayev S, Shvidchenko V, Zotova L, Koekemoer F, De Groot S, Soole K, Langridge P. Early flowering as a drought escape mechanism in plants: how can it aid wheat production? Front Plant Sci. 2017;8:1950.
Schmalenbach I, Zhang L, Reymond M, Jiménez-Gómez JM. The relationship between flowering time and growth responses to drought in the Arabidopsis Landsberg erecta x Antwerp-1 population. Front Plant Sci. 2014;5:609.
Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials: I Spatial distribution of expansive growth. Plant Physiol. 1988;87:50–7.
Bagyaraj DJ, Sharma MP, Maiti D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr Sci. 2015;108:1288–93.
Aliasgharzad N, Neyshabouri MR, Salimi G. Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biologia. 2006;61:S324–8.
Egamberdiyeva D, Qarshieva D, Davranov K. Growth and yield of soybean varieties inoculated with Bradyrhizobium spp. N-deficient calcareous soils. Biol Fert Soils. 2004;40(2):144–6.
Bakr J, Daood HG, Pék Z, Helyes L, Posta K. Yield and quality of mycorrhized processing tomato under water scarcity. Appl Ecol Environ Res. 2017;15(1):401–13.
Oliveira RS, Carvalho P, Marques G, Ferreira L, Pereira S, Nunes M, et al. Improved grain yield of cowpea (Vigna unguiculata) under water deficit after inoculation with Bradyrhizobium elkanii and Rhizophagus irregularis. Crop Pasture Sci. 2017;68(11):1052–9.
Ghorchiani M, Etesami H, Alikhani HA. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric Ecosyst Environ. 2018;258:59–70.
Rahimzadeh S, Pirzad A. Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L): A field study. Mycorrhiza. 2017;27(6):537–52.
Koegel S, Lahmidi NA, Arnould C, Chatagnier O, et al. The family of ammonium transporters (AMT) in Sorghum bicolor: two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2013;198:853–65.
Ortiz N, Armada E, Duque E, Roldán A, Azcón R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J Plant Physiol. 2015;174:87–96.
Impa SM, Nadaradjan S, Boominathan P, Shashidhar G, Bindhumadhava H, Sheshshayee MS. Carbon Isotope Discrimination accurately reflects variability in WUE measured at a whole plant level in rice. Crop Sci. 2005;45:2517–22.
Samarah NH, Alqudah AM, Amayreh JA, McAndrews GM. The effect of late-terminal drought stress on yield components of four barley cultivars. J Agron Crop Sci. 2009;195:427–41.
Wu QS, **a RX. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol. 2006;163(4):417–25.
Augé RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11(1):3–42.
Erman M, Demir S, Ocak E, Tüfenkçi Ş, Oğuz F, Akköprü A. Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions 1-Yield, yield components, nodulation and AM fungi colonization. Field Crops Res. 2011;122:14–24.
Babalola OA, Atayese MO, Soyoye T. Influence of Bradyrhizobium and two Glomus species on the growth and yield of soybean. J Agr Sci Environ. 2009;9:79–95.
Stancheva I, Geneva M, Zehirov G, Tsvetkova G, Hristozkova M, Georgiev G. Effects of combined inoculation of pea plants with arbuscular mycorrhizal fungi and Rhizobium on nodule formation and nitrogen fixing activity. Gen Appl Plant Physiol. 2006;32:61–6.
Ngumbi E, Kloepper J. Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol. 2014;105:109–25.
Braud A, Jezequel K, Bazot S, Lebeau T. Enhanced phytoextraction of an agricultural Cr, Hg and Pb contaminated soil by bioaugmentation with siderophore producing bacteria. Chemosphere. 2009;74:280–6.
Singh M, Mishra M, Srivastava DK, Singh PK. Arbuscular Mycorrhiza-associated rhizobacteria and biocontrol of soilborne phytopathogens. In: Singh M, Mishra M, Srivastava DK, Singh PK, editors. Biostimulants in plant science. London: IntechOpen; 2020. https://doi.org/10.5772/intechopen.89266.
Barea JM, Azcón R, Azcón-Aguilar C. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek. 2002;81:343–51.
Bhattacharyya A, Pablo CH, Mavrodi OV, Weller DM, Thomashow LS, Mavrodi DV. Rhizosphere plant-microbe interactions under water stress. Adv Appl Microbiol. 2021;115:65–113.
Holland EA, Coleman DC. Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology. 1987;68:425–33.
Hungria M, Campo RJ, Mendes IC, Graham PH. Contribution of biological nitrogen fixation to the N nutrition of grain crops in the tropics: the success of soybean (Glycine max L Merr) in South America. In: Singh RP, Shankar N, Jaiwa PK, editors. Nitrogen nutrition and sustainable plant productivity. Houston: Studium Press; 2006. p. 43–93.
Hernández G, Valdés-López O, Ramírez M, Goffard N, Weiller G, Aparicio-Fabre R, et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009;151(3):1221–38.
Rotaru V, Sinclair TR. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ Exp Bot. 2009;66:94–9.
Smith SE, Read DJ. Mycorrhizal symbiosis. London: Academic Press; 2010.
Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall of India; 1973.
Ashwin R, Bagyaraj DJ, Mohan Raju B. Evaluation of different arbuscular mycorrhizal fungi for selecting the best for inoculating soybean cultivars MAUS 2 and MAUS 212. Pertanika J Trop Agri Sci. 2018;41:1587–98.
Ashwin R, Bagyaraj DJ, Mohan RB. Dual inoculation with rhizobia and arbuscular mycorrhizal fungus improves water stress tolerance and productivity in soybean. 2022. Plant Stress. https://doi.org/10.1016/jstress2022100084.
Bagyaraj DJ, Sturmer SL. Arbuscular mycorrhizal fungi (AMF). In: Moreira FMS, Huising JE, Bignell DE, editors. A handbook of tropical soil biology: sampling and characterization of belowground biodiversity. London: Earthscan Pub; 2008. p. 131–47.
Sharma MP, Singh S, Sharma SK, Ramesh A, Bhatia VS. Co-inoculation of resident AM fungi and soybean rhizobia enhanced nodulation, yield, soil biological parameters and saved fertilizer inputs in vertisols under microcosm and field conditions. Soybean Res. 2016;14:39–53.
Shashidhara KN. Identification and validation of high water use efficient and better root type sunflower (Helianthus annuus L) in breds and parental lines for improved drought tolerance. Bangalore: PhD Thesis, UAS, GKVK; 2009.
Pushpa D. Molecular characterisation of the variability in cellular level tolerance in rice (Oryza sativa L): an analysis based on metabolomics approach. Bangalore: PhD Thesis, UAS, GKVK; 2016.
Leopold AC, Musgrave ME, Williams KM. Solute leakage resulting from leaf desiccation. Plant Physiol. 1981;68:1222–5.
Kalina M, Parmer JM. The reduction of tetrazolium salts by mitochondria. Histochemistry. 1986;14:366–74.
Hiscox JD, Israelstam GF. A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–4.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7.
Lowry OH. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Castillo FI, Penel I, Greppin H. Peroxidase release induced by ozone in sedum album leaves. Plant Physiol. 1984;74:846–51.
Davis BJ. Disk electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci. 1964;121:404–27.
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101.
O’Neal ME, Landis DA, Isaacs R. An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Economic Entomol. 2022;95:1190–4.
Hatchell GE, Berry CR, Muse HD. Nondestructive indices related to aboveground biomass of young loblolly and sand pines on ectomycorrhizal and fertilizer plots. For Sci. 1985;31:419–27.
Harrington JT, Mexal JG, Fisher JT. Volume displacement provides a quick and accurate way to quantify new root production. Tree Plant Notes. 1994;45(4):121–4.
Donald CM, Hamblin J. The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Adv Agron. 1976;28:361–405.
Philips JH, Hayman DS. Improved procedures for cleaning roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–61.
Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet-sieving and decanting. Trans Br Mycol Soc. 1963;46:235–44.
Ben-David A, Davidson CE. Estimation method for serial dilution experiments. J Microbiol Methods. 2014;107:214–21.
Casida L, Klein D, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98:371–96.
Buyer JS, Sasser M. High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol. 2012;61:127–30.
Sharma MP, Buyer JS. Comparison of biochemical and microscopic methods for quantification of arbuscular mycorrhizal fungi in soil and roots. Appl Soil Ecol. 2015;95:86–9.
Oliveira SR, Neto JAG, Nóbrega JA, Jones BT. Determination of macro-and micronutrients in plant leaves by high-resolution continuum source flame atomic absorption spectrometry combining instrumental and sample preparation strategies. Spectrochim Acta B At Spectrosc. 2010;65:316–20.
Panse VS, Sukhatme PV. Statistical methods for agricultural workers. 4th ed. New Delhi: ICAR Publication; 1985.
Acknowledgements
The first author is thankful to Dr. Mahaveer P Sharma, ICAR-Indian Institute of Soybean Research, Indore; Dr. Veena S Anil, Dept. of Biotechnology, University of Agricultural Sciences (UAS), Bangalore; Dr. M S Sheshshayee, Dept. of Crop Physiology, UAS, Bangalore for guiding and allowing to analyse few important experimental parameters at their respective labs. The authors are also thankful to Prof. Joanna Dames, Deputy Dean of Science, Rhodes University, Grahamstown/Makhanda, South Africa for English language editing.
Funding
Funded by ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM), Kushmaur, Mau under the scheme ‘Application of Microorganisms in Agriculture and Allied Sectors (AMAAS)’ (Grant No. NBAIM/AMAAS/2014-17/PF/48).
Author information
Authors and Affiliations
Contributions
AR: data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft; DJB: conceptualization, funding acquisition, methodology, project administration, resources, supervision, review and editing; BMR: conceptualization, methodology, resources, supervision, validation, review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The whole study and experimental analysis was conducted by the first author Ashwin Revanna at CNBRCD, Bangalore, India for his Doctor of Philosophy degree registered at PRIST University, Thanjavur, Tamil Nadu, India under the mentorship of Dr. D. J. Bagyaraj and Dr. Mohan Raju B.
Competing interests
All the authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Fig. S1. Influence of dual inoculation on a soil moisture, b soil water potential and c soil temperature of study plots analyzed at flowering and pod filling stage in a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions. Dual inoculation: Ambispora leptoticha + Bradyrhizobium liaoningense; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress, Flowering stage: 1st stress period (35-60 DAS); Pod filling stage: 2nd stress period (85-100 DAS); No significant differences (p ≤ 0.05) observed relative to controls UI & UIS and their respective treatments.
Additional file 2
: Fig. S2. Influence of dual inoculation on a SPAD chlorophyll meter reading (SCMR) value, b Total chlorophyll content and c Chlorophyll stability index of leaves at flowering and pod filling stage in a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions. Dual inoculation: Ambispora leptoticha + Bradyrhizobium liaoningense; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress, Flowering stage: 1st stress period (35-60 DAS), Pod filling stage: 2nd stress period (85-100 DAS); Significant differences (p ≤ 0.05) relative to controls UI & UIS are indicated by asterisk (*) & hash (#) respectively.
Additional file 3
: Fig. S3. Comparison of leaf growth in soybean cultivar, MAUS 2 inoculated with Ambispora leptoticha + Bradyrhizobium liaoningense grown under irrigated and moisture stressed field conditions.
Additional file 4
: Table S1. Influence of dual inoculation on plant height, stem diameter, biovolume index and flowering in a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions.
Additional file 5
: Table S2. Influence of dual inoculation on number of nodules, nodule weight, mycorrhizal spore numbers in the root zone soil and the percent mycorrhizal root colonization in a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions.
Additional file 6
: Table S3. Influence of dual inoculation on rhizosphere microbial communities and dehydrogenase activity before and after imposing the stress in a drought susceptible soybean cultivar, MAUS 2 grown under irrigated and moisture stressed field conditions
Additional file 7
: Fig. S4. a Principal Component analysis of the PLFA biomarkers from the rhizosphere soils of soybean cultivar, MAUS 2 inoculated with Ambispora leptoticha + Bradyrhizobium liaoningense. b PLFA variables subjected to redundancy analysis by PCA. [10 PLFA biomarkers (including 2 ratios) were selected for the PCA model where axis labels indicate variables that were strong negative or positive factors on each axis (biomarker- Fungi, Gram −ve, Eukaryote contributed negatively]; UI un-inoculated, I inoculated, UIS un-inoculated stress, IS inoculated stress.
Additional file 8
: Table S4. Summary of crop** and treatment details.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashwin, R., Bagyaraj, D.J. & Mohan Raju, B. Ameliorating the drought stress tolerance of a susceptible soybean cultivar, MAUS 2 through dual inoculation with selected rhizobia and AM fungus. Fungal Biol Biotechnol 10, 10 (2023). https://doi.org/10.1186/s40694-023-00157-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40694-023-00157-y