Abstract
Background
The Response Evaluation Criteria in Solid Tumors (RECIST) are often inadequate for the early assessment of the response to cancer therapy, particularly bevacizumab-based chemotherapy. In a first cohort of patients with colorectal cancer liver metastases (CRLM), we showed that variations of the tumor-to-liver density (TTLD) ratio and modified size-based criteria determined using computed tomography (CT) data at the first restaging were better prognostic criteria than the RECIST. The aims of this study were to confirm the relevance of these radiological biomarkers as early predictors of the long-term clinical outcome and to assess their correlation with contrast-enhanced ultrasound (CEUS) parameters in a new patient cohort.
Methods
In this post-hoc study of the multicenter STIC-AVASTIN trial, we retrospectively reviewed CT data of patients with CRLM treated with bevacizumab-based regimens. We determined the size, density and TTLD ratio of target liver lesions at baseline and at the first restaging and also performed a morphologic evaluation according to the MD Anderson criteria. We assessed the correlation of these parameters with progression-free survival (PFS) and overall survival (OS) using the log-rank test and a Cox proportional hazard model. We also examined the association between TTLD ratio and quantitative CEUS parameters.
Results
This analysis concerned 79 of the 137 patients included in the STIC-AVASTIN trial. PFS and OS were significantly longer in patients with tumor size reduction > 15% at first restaging, but were not correlated with TTLD ratio variations. However, PFS was longer in patients with TTLD ratio > 0.6 at baseline and first restaging than in those who did not reach this threshold. In the multivariate analysis, only baseline TTLD ratio > 0.6 was a significant survival predictor. TTLD ratio > 0.6 was associated with improved perfusion parameters.
Conclusions
Although TTLD ratio variations did not correlate with the long-term clinical outcomes, TTLD absolute values remained a good predictor of survival at baseline and first restaging, and may reflect tumor microvascular features that might influence bevacizumab-based treatment efficiency.
Trial registration
NCT00489697, registration number of the STIC-AVASTIN trial.
Similar content being viewed by others
Introduction
Colorectal cancer remains one of the most common and aggressive cancers in Europe, with approximately 500,000 new cases in 2018 [1]. Most patients will develop metastases that are often disseminated and unresectable [2]. The current guidelines recommend upfront systemic combination treatments with cytotoxic chemotherapy and biologic agents that inhibit mechanisms associated with cancer progression (e.g. angiogenesis inhibitors) [3,4,5]. Bevacizumab, the first approved antiangiogenic drug, is a monoclonal antibody that binds to and antagonizes vascular endothelial growth factor (VEGF) A, a key tumor angiogenesis factor. The combination of fluoropyrimidine-based chemotherapy plus oxaliplatin and/or irinotecan with bevacizumab as first- or second-line treatment for metastatic disease significantly improves patient outcomes in bevacizumab-naive patients [6,7,8]. The combination of bevacizumab and fluoropyrimidine also provides a survival benefit as maintenance therapy in patients with good response to a more intensive initial treatment, as demonstrated by the phase III CAIRO3 trial [9]. Furthermore, Bennouna et al. showed that bevacizumab continuation with a second-line chemotherapy regimen after the first progression prolonged overall survival (OS) compared to chemotherapy alone. This validated the concept of the “continuous anti-angiogenic blocking” approach [10]. However, although bevacizumab is now routinely used, there is no robust predictive marker to identify the patients who are more likely to benefit from angiogenesis inhibitors [11]. Moreover, bevacizumab efficacy is not always associated with tumor shrinkage [12]. The standard Response Evaluation Criteria in Solid Tumors (RECIST), based on tumor long axis measurements in axial computed tomography (CT) images, may be inadequate for the early assessment of bevacizumab efficacy. Therefore, alternative radiologic biomarkers have been investigated. Several studies using functional imaging modalities, such as contrast-enhanced ultrasound (CEUS), found a correlation between patient outcomes and early changes in liver tumor perfusion parameters in response to anti-VEGF pathway agents [13, 14]. Similarly, in patients with colorectal cancer liver metastases (CRLM) treated with bevacizumab before surgical resection, optimal morphologic response (i.e. the transformation into lesions with a homogeneous overall attenuation and a sharp tumor-liver interface), but not the RECIST, was associated with a better pathologic response and better outcomes [15]. The on-treatment early prognostic value of the morphologic response was subsequently confirmed in patients with unresectable CRLM [16,17,18].
Recently, we developed an alternative method based on tumor lesion size and attenuation assessment that can be easily performed using standard portal venous phase CT images. We showed that in CRLM, progression-free survival (PFS) and OS are significantly longer in patients with tumor size reduction > 15% and/or a tumor-to-liver density (TTLD) ratio variation not lower than -10% at the first restaging CT after the initiation of first-line combination therapy with XELIRI or FOLFIRI [19].
The aim of this post-hoc study was to confirm our first results in an independent population with CRLM treated with chemotherapy plus bevacizumab. We also evaluated the correlation between TTLD and CEUS parameters to better understand how bevacizumab provides its real benefit.
Material and methods
Study design
We performed a post-hoc analysis of contrast-enhanced CT (CECT) images from a prospectively accrued cohort of 137 patients with unresectable CRLM treated with bi-weekly bevacizumab-based chemotherapy as first-line therapy in a French multicenter noncomparative trial (NCT00489697, ID number INCA06-FT/STIC-AVASTIN) from January 2007 to November 2010. The objective of the main study was to assess the correlation between liver CEUS parameters and radiological response and outcome [14]. Patients underwent CT imaging with multidetector row helicoidal acquisitions before treatment and at day 60 after treatment start, then every 8 or 12 weeks at the investigator’s discretion.
Patient selection
Patients with at least one liver metastasis larger than 1.5 cm, with baseline and restaging CT data of sufficient quality to allow assessing the density response, and with images acquired during the portal venous phase following intravenous contrast injection with a slice thickness < 3 mm were included in this post-hoc study. The contrast enhancement quality was assessed by analyzing portal and hepatic vein attenuation. All CECT images with a vascular density < 100 Hounsfield Units (HU) were excluded from the analysis.
Image analysis
All CT images used in this study were anonymized. Target liver lesions on the pretreatment (baseline) CT were measured manually as well as the long axis diameters according to the RECIST 1.1. A maximum of two metastases were selected in patients with multiple tumor lesions. Then, volumetric segmentation of the tumor was performed using a semi-automated edge detection software (Myrian®, Intrasense, Montpellier, France). Tumor edges were adjusted until a satisfactory three-dimensional selection of a target lesion was obtained, and then the mean tumor volumetric attenuations were measured. The software also automatically segmented the healthy liver, excluding metastases and hepatic vessels, to calculate the mean density. To compensate for intra- and inter-individual heterogeneity in liver contrast enhancement, the TTLD ratio was determined, i.e. the mean tumor density divided by the mean healthy liver density. The same analysis was performed using CT images obtained at the first restaging. To minimize inter-observer variability, target liver lesion selection, size measurement and segmentation were performed by a single operator (T.M., GI oncologist) trained in the use of the software and involved in a previous study using this approach [19]. Whenever possible, the radiologist (E.L.) who contributed to develop the MD Anderson criteria [15] also assessed the morphologic response.
CEUS parameters
When available, the relative values (i.e. compared to healthy liver, expressed in percentage) of 11 CEUS parameters determined at day 0 (D0) and day 60 (D60) were obtained from the main study: peak enhancement (PE), area under the curve during wash-in (WiAUC) and wash-out (WoAUC), total area under the curve (AUC = WiAUC + WoAUC), time-to-peak (TTP), rise time (RT), fall time (FT), wash-in (WiR) and wash-out (WoR) rates, wash-in perfusion index (WiPI), and mean transit time (mTT).
Statistical analysis
Categorical variables were reported as numbers and frequencies. Continuous variables were reported as median and range and compared with the Wilcoxon rank-sum test.
The mean tumor density was evaluated using a threshold of 63 HU to stratify patients in accordance with the results by Dohan et al. [20]. The TTLD ratio variations were analyzed using a -10% cut-off, as established in our previous work [19]. Moreover, the median TTLD ratio at baseline was used as a categorizer. Modified RECIST (RECIST-15) were used for size reduction at the first restaging, with response defined as a > 15% reduction in the sum of target liver lesions.
PFS was the primary endpoint, defined as the time from the baseline CT exam to the date of the first disease progression or death from any cause. Patients alive without disease progression were censored at the date of the last visit. OS was the secondary endpoint, defined as the time from the baseline CT exam to death from any cause. The median follow-up was calculated using the Schemper method. PFS and OS were estimated using the Kaplan–Meier method and reported as median or rate at specific time points with their 95% confidence intervals (CI).
A Cox proportional hazard model was used to estimate the hazard ratios (HR) with their 95%CI for the prognostic factors associated with PFS and OS. Variables with p-values < 0.15 in the univariate analysis were retained for the multivariate analysis and a backward covariate selection was performed.
Results
Population
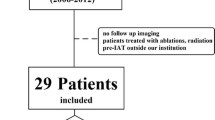
In total, 79 of the 137 patients enrolled in the main trial were eligible for this post-hoc analysis. The reasons for exclusion are listed in Fig. 1. Their clinical characteristics and outcomes were similar to those of the whole population (data not shown). Table 1 shows the characteristics of the 79 patients.
Radiological parameters
The median (range) time from baseline CT to the first restaging CT was 2.3 (1.6–3.4) months. The median PFS and OS were 11 (95% CI: 9.4, 12.2) and 25.1 months (95% CI: 21.2, 31.8), respectively, and the median follow-up was 50 months (1.9–64.2). The baseline mean tumor density ranged from 23.9 to 117.3 HU and the median value was 62.8 HU. At the first restaging, the mean tumor density ranged from 6.9 to 179.5 HU and the median value was 53.5 HU. The median TTLD ratio was 0.62 at baseline and 0.54 at the first restaging (Table 2).
At the first restaging, according to the RECIST-15, 63%, 34%, and 3% of patients were classified as having a complete/partial response, stable disease, or progressive disease, respectively. Moreover, 31 (47%) patients achieved an optimal morphologic response, according to the MD Anderson morphologic criteria.
Associations between quantitative CT parameters and survival
Four variables were identified as significant (p < 0.05) prognostic parameters of PFS in the univariate analysis: time of metastasis diagnosis (synchronous vs. metachronous; HR = 0.57, 95% CI: 0.32- 1.00), RECIST-15 value at the first restaging (≥ -15% vs. < -15%; HR = 1.77, 95% CI: 1.09–2.88), and TTLD ratio at baseline (> 0.6 vs. ≤ 0.6; HR = 0.53, 95% CI: 0.33–0.86) and first restaging (> 0.6 vs. ≤ 0.6; HR = 0.58, 95% CI: 0.35–0.96). Moreover, PFS tended to be shorter in patients with non-optimal morphologic response (2–3 vs. 1; HR = 1.44, 95% CI: 0.88–2.37). Therefore, this parameter was kept for the multivariate analysis. In the multivariate analysis, only baseline TTLD ratio (> 0.6 vs. ≤ 0.6; HR = 0.51, 95% CI: 0.29–0.88) and morphologic response according to the MD Anderson criteria (1 vs. 2–3; HR = 1.79, 95% CI: 1.05–3.05) remained significant (Table 3).
In the univariate analysis, OS was correlated with baseline TTLD ratio (> 0.6 vs. ≤ 0.6; HR = 0.55, 95% CI: 0.33, 0.92), RECIST-15 value at the first restaging (≥ -15% vs. < -15%; HR = 1.79, 95% CI: 1.06, 3.00), and date of metastasis diagnosis (synchronous vs. metachronous; HR = 0.52, 95% CI: 0.28–0.99), but not with TTLD ratio at the first restaging and morphologic response. In the multivariate analysis, only the RECIST-15 value (≥ -15% vs. < -15%; HR = 1.75, 95% CI: 1.05–2.94) and time of metastasis diagnosis (synchronous vs. metachronous; HR = 0.53, 95% CI: 0.28–1) remained significant (Table 4).
Correlation between CT and quantitative CEUS parameters
When patients were divided in two groups based on the median TTLD ratio at baseline (i.e. 0.6), survival (particularly PFS) was correlated with the TTLD ratio at baseline and at the first restaging. Therefore, quantitative CEUS parameters at D0 and D60 were compared in these two patient groups. At both time points, almost all blood volume parameters were significantly higher in patients with TTLD ratio > 0.6. Among the blood flow parameters, only WiAUC, WiPI and WoR rates (both time points) and mTT (D60) were significantly higher in the TTLD ratio > 0.6 group (Table 5). Fig. 2 illustrates a liver metastasis with a high TTLD ratio before and after treatment on the CT scan and a high peak enhancement measured by CEUS at D0.
Liver metastasis with a high TTLD ratio with correlative CEUS images and data. Example of CT images acquired during the portal venous phase showing a metastasis with a TTLD ratio > 0.6 at baseline (A) and at first restaging (B). Late phase correlative CEUS image of the same lesion captured at baseline after SonoVue® injection with ROI drawing around the metastatic lesion delineated in green and the reference normal liver tissue in yellow (C). Time-intensity curve of both ROIs with absolute values of peak enhancement and value relative to the healthy liver (in percent, determined by the ratio lesion/reference values *100) (D)
Discussion
Our study demonstrates that alternative radiological parameters may provide useful prognostic information in patients with CRLM receiving bevacizumab-based first-line therapy. High TTLD ratio was independently associated with better outcome and may reflect a tumor vasculature that is more likely to benefit from anti-angiogenic therapies, as suggested by its correlation with perfusion parameters quantified by CEUS.
More than 15 years after bevacizumab implementation for the routine management of metastatic colorectal cancer, biomarkers are still missing to improve patient selection before treatment and to assess its specific efficacy during treatment. Several circulating and tissue components implicated in angiogenesis have been evaluated, including VEGF. However, to date, they have only shown a prognostic impact and have not been integrated into the “clinical decision making” [11, 21,22,23,24]. This can be explained by different reasons. First, angiogenesis mechanisms are very complex and involve a large number of factors that are difficult to address holistically. In addition, bevacizumab is systematically associated with cytotoxic chemotherapy, thus preventing the study of its specific effect. Moreover, the methods used to measure these markers are often not reproducible and several ancillary studies lacked a control group to assess the predictive value [25]. Therefore, an alternative approach has been to assess directly bevacizumab effect on the tumor neo-vasculature using static imaging, mainly by exploiting additional features from the cross-sectional images obtained during routine CT, or dynamic imaging, mainly using functional imaging methods. Based on this alternative approach, here, we assessed the prognostic value of the TTLD ratio, which can be easily measured on portal phase CECT images with a dedicated software, and of a modified, size-based RECIST criterion [19]. We confirmed that PFS and OS were longer in patients with a decrease in the total sum of target liver lesions > 15%. This is consistent with previous studies showing that an early size shrinkage with a less stringent threshold (15 or 20%) than the one used in the RECIST 1.1 may be a surrogate marker of clinical outcome in patients with metastatic colorectal cancer, including those receiving antiangiogenic chemotherapy [20, 26, 27].
Unlike in our previous study [19], the TTLD ratio change between baseline and the first restaging using a threshold of -10% did not predict patient outcome. However, the TTLD ratio at specific time points was still correlated with survival. Specifically, in patients with a TTLD ratio > 0.6 at baseline and at the first restaging, the risk of progression was significantly reduced at both time points. This correlation remained significant in the multivariate analysis only for the baseline TTLD ratio. We observed the same trend for OS, although it was not significant in the multivariate analysis. We also found differences in quantitative CEUS parameters between patients with TTLD ratio > 0.6 and ≤ 0.6. This may provide a more comprehensive understanding of this candidate surrogate marker. Indeed, blood volume parameters and some of the blood flow parameters were higher in patients with TTLD ratio > 0.6 than with TTLD ≤ 0.6 at baseline and the first restaging. Liver CEUS was developed to better detect and characterize focal lesions through the qualitative assessment of the vascular architecture and phase-specific contrast enhancement relative to the adjacent healthy liver parenchyma. It also allows the quantitative assessment of the solid tumor perfusion using a time-intensity curve that represents the transition of the contrast agent in the region of interest. Moreover, the ultrasound contrast agents used in CEUS remain purely intravascular, unlike those used in CECT and contrast-enhanced MRI. Thus, the data obtained with this technique only model the flow of the contrast microbubbles in the micro- and macro-vasculature of the selected region of interest [28, 29]. Several studies evaluated CEUS parameters in patients with metastatic colorectal cancer treated with bevacizumab-based chemotherapy. Overall, they showed a decrease in the perfusion parameters during the first weeks of therapy, besides some blood flow characteristics. This suggests a bevacizumab anti-angiogenic mechanism of vascular pruning to starve the tumor. These studies also reported that variations in CEUS parameters could sometimes predict survival; however, the optimal cut-offs were inconsistent among studies and pre-treatment parameters were not prognostic [13, 14, 30,31,32,33,34]. Conversely, our study suggests that the patients with tumors that displayed higher perfusion parameters at baseline and that retained a sufficient degree of perfusion, albeit reduced, after treatment have the best prognosis. These results are consistent with the hypothesis made by Jain et al. on the vascular normalizing effect of anti-angiogenic drugs and its prerequisites to alleviate hypoxia [35, 36]. First, a “minimal” tumor vasculature is needed before treatment initiation because the increase of functional vessels cannot overcome the paucity of vessels. Previous studies suggested that tumors with higher baseline microvascular density or higher surrogate markers of tumor microvascular density (such as CT attenuation) are most likely to benefit from anticancer treatments, including bevacizumab [20, 37, 38]. Second, excessive pruning should be avoided during treatment to preserve the benefit of the improved function of normalized vessels, as suggested by the results of an exploratory correlative study of serial biopsies from patients with localized breast cancer receiving bevacizumab-based neoadjuvant chemotherapy [35]. Pre-clinical and clinical studies by Jain’s group confirmed the vasculature-normalizing effect of anti-angiogenic therapies in solid tumors and in non-malignant diseases [39,40,41,42]. In a rabbit model of tuberculosis, they repurposed bevacizumab as a host-directed therapy that resulted in the reduction in vessel number and the increase in vessel pericyte coverage and lumen area. This led to improved drug delivery and oxygenation in lung granulomas, which are characteristic of this infection and share abnormal microenvironment features with solid tumors [43].
This study also provided the opportunity to re-evaluate in an independent cohort of patients with CRLM the performance of the morphological response according to the MD Anderson criteria, another recently proposed CT imaging marker to predict the early clinical response to bevacizumab-based treatment. Similarly to previous studies in cohorts of patients with unresectable metastatic colorectal cancer, this marker was independently associated with longer PFS [16,17,18] but not with OS, as confirmed by Dohan et al. in a large cohort of patients treated with first-line FOLFIRI plus bevacizumab [20]. Morphologic criteria are simple to measure, require a short learning curve and show a strong interobserver agreement, especially to accurately identify optimal responders and non-responders [15, 20, 44, 45]. However, they are underused, possibly because of the reluctance to use a subjective method and the limitations of suboptimal CT techniques.
The TTLD ratio is more objective, but has several limitations. First, it provides only a rough and global assessment of the metastases. For example, it does not take into account the heterogeneity of the intra-tumoral vessel distribution and of the inter-tumoral response. Moreover, as the microvascular volume represents only 10% of the whole tumor volume, this ratio also takes into account characteristics of the non-vascular compartment [46]. However, this could be an advantage because it could be used as a surrogate marker for other components of the tumor microenvironment that may positively or negatively influence the treatment efficacy. Future studies should assess the correlation with histological tissue features. In addition, part of the target lesions selected for the CT analysis were not necessarily the same as those selected for the ultrasound analysis. Eventually, currently, the TTLD ratio seems to correlate mainly with microvascular density but it is not indicative of their functionality, unlike dynamic contrast-enhanced MRI and perfusion CT that can assess the capillary permeability.
This study has some limitations, particularly the small sample size that restricted the statistical analysis. However, this is the first study that assessed the correlation of different radiologic biomarkers to better understand bevacizumab activity. Two other limitations are the absence of a control group to assess their predictive value of the bevacizumab activity, and the inclusion of patients who received different chemotherapy regimens, although irinotecan-based chemotherapy was the most frequent.
The identification of predictive biomarkers of anti-angiogenic drug efficacy should remain a priority in the next years. These agents are expected to play a significant role especially in the management of patients with metastatic colorectal cancer. For instance, following the results of the randomized trial SUNLIGHT, bevacizumab is now indicated, in combination with trifluridine-tipiracil (FTD-TPI), in patients with refractory metastatic colorectal cancer. Indeed, Prager et al. demonstrated that bevacizumab addition to FTD-TPI improves OS compared to FTD-TPI alone [47]. Moreover, fruquintinib, a highly selective oral inhibitor of the VEGF receptors 1, 2, and 3, is now introduced in daily practice as it showed significant benefits compared with placebo in heavily pretreated patients in the FRESCO 2 phase III study [48]. However, although the effectiveness of prolonged angiogenic inhibition is confirmed, the therapeutic benefit is limited to only few months and tumor shrinkage is rare. In addition, there are ongoing studies to repurpose bevacizumab and other anti-angiogenic molecules in combination with immunotherapy since combining immune and vascular modulation is a promising way to improve cancer therapy. Indeed, the abnormal tumor angiogenesis also promotes immunosuppressive functions [49]. On the other hand, different immune cells, such as CD8 + and CD4 + T cells and eosinophils, can contribute to vascular normalization [50]. However, so far, this combination has given very contrasting results in gastrointestinal malignancies. For instance, the combination of the immune checkpoint inhibitor (ICI) atezolizumab with bevacizumab has become the standard first-line treatment in advanced hepatocellular carcinoma [51]. Conversely, a recent study showed that the combination of the ICI pembrolizumab with lenvatinib, another VEGF receptor inhibitor, did not improve OS compared to the standard of care in 480 patients with previously treated metastatic colorectal cancer who were unselected for microsatellite instability high/mismatch repair deficiency [52].
We think that imaging approaches have undeniable advantages to identify the best responders to these different strategies. They are non-invasive, repeatable and provide a more comprehensive view of the tumor and its microenvironment compared with blood biomarkers, which are always affected by systemic dilution due to the influence of normal tissues or the need for a multi-analytic approach given the complexity of the studied phenomena. Although our approach may be considered simple, it lays the groundwork for the future development of an optimized radiological marker for vascular or microenvironment normalization. For example, it would be interesting to determine whether the TTLD ratio correlates with the ICI lack of efficacy frequently observed in patients with CRLM [53, 54]. Lastly, significant advances could also come from the development of more sophisticated image analysis systems based on radiomics and artificial intelligence. These systems have recently shown very promising results in patients with gastrointestinal cancer [55, 56].
Conclusions
In this study, we showed that in patients with CRLM, clinical outcome correlated with early tumor shrinkage, but not with TTLD ratio variations. Nevertheless, the TTLD ratio absolute value, especially at baseline, may predict the efficacy of bevacizumab-based treatment by reflecting tumor microenvironment features that may influence its effect.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87.
Benson AB, Hoffe S, Nurkin S, Overman MJ. NCCN Guidelines Index Table of Contents. 2018;176.
Phelip JM, Tougeron D, Léonard D, Benhaim L, Desolneux G, Dupré A, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51:1357–63.
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–59.
Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;S0923753422041928.
Hurwitz H, Fehrenbacher L, Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;2335–42.
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the eastern cooperative oncology group study E3200. J Clin Oncol. 2007;25:1539–44.
Simkens LHJ, van Tinteren H, May A, ten Tije AJ, Creemers GJM, Loosveld OJL, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch colorectal cancer group. Lancet. 2015;385:1843–52.
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Angelucci A, Delle Monache S, Cortellini A, Di Padova M, Ficorella C. “Vessels in the Storm”: searching for prognostic and predictive angiogenic factors in colorectal cancer. Int J Mol Sci. 2018;19:299.
Grothey A, Hedrick EE, Mass RD, Sarkar S, Suzuki S, Ramanathan RK, et al. Response-Independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008;26:183–9.
Lassau N, Coiffier B, Kind M, Vilgrain V, Lacroix J, Cuinet M, et al. Selection of an early biomarker for vascular normalization using dynamic contrast-enhanced ultrasonography to predict outcomes of metastatic patients treated with bevacizumab. Ann Oncol. 2016;27:1922–8.
Tranquart F, Dujardin P-A, Bouché O, Marcus C, Borg C, Manzoni P, et al. Value of Contrast-Enhanced Ultrasound Quantification Criteria for Identifying Patients not Responding to Bevacizumab-Based Therapy for Colorectal Liver Metastases. Ultraschall Med - Eur J Ultrasound. 2017 [cited 2018 Aug 31]; Available from: http://www.thieme-connect.de/DOI/DOI?https://doi.org/10.1055/s-0043-122497
Chun Y, Vauthey J, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–44.
Yoshita H, Hosokawa A, Ueda A, Ando T, Kajiura S, Kato H, et al. Predictive value of optimal morphologic response to first-line chemotherapy in patients with colorectal liver metastases. Digestion. 2014;89:43–8.
Mazard T, Boonsirikamchai P, Overman MJ, Asran MA, Choi H, Herron D, et al. Comparison of early radiological predictors of outcome in patients with colorectal cancer with unresectable hepatic metastases treated with bevacizumab. Gut. 2018;67:1095–102.
Masuishi T, Taniguchi H, Eto T, Komori A, Mitani S, Hasegawa H, et al. Morphologic response and tumor shrinkage as early predictive markers in unresectable colorectal liver metastases. Anticancer Res. 2018;38:6501–6.
Mazard T, Assenat E, Dupuy M, Mollevi C, René A, Adenis A, et al. Quantitative evaluation of liver metastases density on computed tomography: A new tool to evaluate early response to bevacizumab-containing chemotherapy. Dig Liver Dis. 2019;S1590865819305341.
Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P, et al. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2019;gutjnl-2018–316407.
Bruhn MA, Townsend AR, Khoon Lee C, Shivasami A, Price TJ, Wrin J, et al. Proangiogenic tumor proteins as potential predictive or prognostic biomarkers for bevacizumab therapy in metastatic colorectal cancer: proangiogenic proteins and bevacizumab therapy in mCRC. Int J Cancer. 2014;135:731–41.
Jary M, Lecomte T, Bouché O, Kim S, Dobi E, Queiroz L, et al. Prognostic value of baseline seric Syndecan-1 in initially unresectable metastatic colorectal cancer patients: a simple biological score. Int J Cancer. 2016;139:2325–35.
Lereclus E, Tout M, Girault A, Baroukh N, Caulet M, Borg C, et al. A possible association of baseline serum IL-17A concentrations with progression-free survival of metastatic colorectal cancer patients treated with a bevacizumab-based regimen. BMC Cancer. 2017;17:220.
Ortiz-Morales MJ, Toledano-Fonseca M, Mena-Osuna R, Cano MT, Gómez-España A, la Haba-Rodríguez JRD, et al. Basal VEGF-A and ACE Plasma Levels of Metastatic Colorectal Cancer Patients Have Prognostic Value for First-Line Treatment with Chemotherapy Plus Bevacizumab. Cancers. 2022;14:3054.
Lambrechts D, Lenz H-J, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–30.
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188–94.
Hosokawa A, Yamazaki K, Matsuda C, Ueda S, Kusaba H, Okamura S, et al. Morphologic response to chemotherapy containing bevacizumab in patients with colorectal liver metastases: a post hoc analysis of the WJOG4407G phase III study. Medicine (Baltimore). 2020;99.
Dietrich C, Averkiou M, Correas J-M, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med - Eur J Ultrasound. 2012;33:344–51.
Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and good clinical practice recommendations for Contrast-Enhanced Ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579–604.
Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, et al. Validation of Dynamic Contrast-Enhanced Ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the french multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794–800.
Mogensen M, Hansen M, Henriksen B, Axelsen T, Vainer B, Osterlind K, et al. Dynamic Contrast-Enhanced Ultrasound of colorectal liver metastases as an imaging modality for early response prediction to chemotherapy. Diagnostics. 2017;7:35.
Amadori M, Barone D, Scarpi E, Oboldi D, Amadori E, Bandi G, et al. Dynamic Contrast-Enhanced Ultrasonography (D-CEUS) for the early prediction of bevacizumab efficacy in patients with metastatic colorectal cancer. Eur Radiol. 2018;28:2969–78.
Wu Z, Yang X, Chen L, Wang Z, Shi Y, Mao H, et al. Anti-angiogenic therapy with contrast-enhanced ultrasound in colorectal cancer patients with liver metastasis. Medicine (Baltimore). 2017;96:e6731.
Schirin-Sokhan R. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol. 2012;18:541.
Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci. 2015;112:14325–30.
Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22.
Froelich MF, Heinemann V, Sommer WH, Holch JW, Schoeppe F, Hesse N, et al. CT attenuation of liver metastases before targeted therapy is a prognostic factor of overall survival in colorectal cancer patients. Results from the randomised, open-label FIRE-3/AIO KRK0306 trial. Eur Radiol. 2018;28:5284–92.
Foernzler D, Delmar P, Kockx M, Cassidy J, Saltz L, Scherer S. Tumor tissue based biomarker analysis in NO16966: A randomized phase III study of first-line bevacizumab in combination with oxaliplatin-based chemotherapy in patients with mCRC. 2010 Gastrointest Cancers Symp Abstr. 2010.
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7.
Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Med Sci.
Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Med Sci.
Boucher Y, Kumar AS, Posada JM, G**i E, Pfaff K, Lipschitz M, et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. NPJ Precis Oncol. 2021;5:62.
Datta M, Via LE, Kamoun WS, Liu C, Chen W, Seano G, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Med Sci.
Nishioka Y, Shindoh J, Yoshioka R, Gonoi W, Abe H, Okura N, et al. Radiological Morphology of Colorectal Liver Metastases after Preoperative Chemotherapy Predicts Tumor Viability and Postoperative Outcomes. J Gastrointest Surg. 2015;1–9.
Wesdorp NJ, Kemna R, Bolhuis K, van Waesberghe JHTM, Nota IMGC, Struik F, et al. Interobserver variability in CT-based morphologic tumor response assessment of colorectal liver metastases. Radiol Imaging Cancer. 2022;4:e210105.
Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol. 2007;8:245–55.
Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388:1657–67.
Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402:41–53.
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40.
Choi Y. Normalization of the tumor microenvironment by harnessing vascular and immune modulation to achieve enhanced cancer therapy. Mol Med. 2023;
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Kawazoe A, Xu R, Passhak M, Teng H, Shergill A, Gumus M, et al. LBA-5 Lenvatinib plus pembrolizumab versus standard of care for previously treated metastatic colorectal cancer (mCRC): the phase 3 LEAP-017 study. Ann Oncol. 2023;34:S179.
Fakih M. Immunotherapy response in microsatellite stable metastatic colorectal cancer is influenced by site of metastases. Eur J Cancer. 2024;
Saberzadeh-Ardestani B. Metastatic site and clinical outcome of patients with deficient mismatch repair metastatic colorectal cancer treated with an immune checkpoint inhibitor in the first-line setting. Eur J Cancer. 2024;
Wesdorp NJ, Hellingman T, Jansma EP, van Waesberghe J-HTM, Boellaard R, Punt CJA. Advanced analytics and artificial intelligence in gastrointestinal cancer: a systematic review of radiomics predicting response to treatment. Eur J Nucl Med Mol Imaging. 2021;48:1785–94.
Mansur A, Saleem Z, Elhakim T, Daye D. Role of artificial intelligence in risk prediction, prognostication, and therapy response assessment in colorectal cancer: current state and future directions. Front Oncol. 2023;13:1065402.
Acknowledgements
We thank all the patients for their participation in the study. We also thank Dr Fanny Salasc of the Montpellier Cancer Institute for providing medical writing and editorial support and Dr Elisabetta Andermarcher for editing the manuscript. This research was supported by the SIRIC Montpellier Cancer Grant INCa_DGOS_INSERM ITMO Cancer_18004.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: TM, CM, EA, TL. Acquisition of data: TM, EML, JL, RC, OB, CB, PAD, AB, TL. Analysis and interpretation of data: TM, CM, EA, TL. Drafting of the manuscript: TM, CM, EA, TL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The main STIC-AVASTIN trial was a French multicenter, non-comparative study (NCT00489697, ID number INCA06-FT/STIC-AVASTIN). This study was approved by Tours Ethics Committee (2006-R10) and the Institutional Review Board. No additional approval was required for the post hoc analysis.
Consent for publication
Not applicable.
Competing interests
TM received honoraria for speaking or consulting roles from Servier, Pierre Fabre, Merck Serono, AAA, Sanofi, Galapagos, and research grants from Amgen and Roche. He also received travel grants from Pierre Fabre, Merck Serono, and Sanofi. OB received honoraria for speaking or consulting roles from Apmonia Therapeutics, Merck KGaA, Bayer, MSD, Amgen, Deciphera, Servier, and Pierre Fabre, outside the submitted work. TL received honoraria for speaking or for consulting roles from Servier, Pierre Fabre, Merck Serono, BMS, Astra Zeneca, AAA, Sanofi, IPSEN, Novartis, CHUGAI outside the submitted work; research grants from Pierre Fabre and Leo Pharma outside the submitted work; and travel grants from IPSEN, Merck Serono, AMGEN, Astra Zeneca, Pierre Fabre and Servier outside the submitted work. EA received honoraria for speaking or consulting roles from Roche, Novartis, MSD, Astra Zeneca, Bayer, Ipsen, Boston, Incyte, Servier, AAA. CM, EML, JL, RC, CB, PAD, and AB declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mazard, T., Mollevi, C., Loyer, E.M. et al. Prognostic value of the tumor-to-liver density ratio in patients with metastatic colorectal cancer treated with bevacizumab-based chemotherapy. A post-hoc study of the STIC-AVASTIN trial. Cancer Imaging 24, 77 (2024). https://doi.org/10.1186/s40644-024-00722-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-024-00722-7