Abstract
Background
Optimal anticoagulation strategies for COVID-19 patients with the acute respiratory distress syndrome (ARDS) on venovenous extracorporeal membrane oxygenation (VV ECMO) remain uncertain. A higher incidence of intracerebral hemorrhage (ICH) during VV ECMO support compared to non-COVID-19 viral ARDS patients has been reported, with increased bleeding rates in COVID-19 attributed to both intensified anticoagulation and a disease-specific endotheliopathy. We hypothesized that lower intensity of anticoagulation during VV ECMO would be associated with a lower risk of ICH. In a retrospective, multicenter study from three academic tertiary intensive care units, we included patients with confirmed COVID-19 ARDS requiring VV ECMO support from March 2020 to January 2022. Patients were grouped by anticoagulation exposure into higher intensity, targeting anti-factor Xa activity (anti-Xa) of 0.3–0.4 U/mL, versus lower intensity, targeting anti-Xa 0.15–0.3 U/mL, cohorts. Mean daily doses of unfractionated heparin (UFH) per kg bodyweight and effectively measured daily anti-factor Xa activities were compared between the groups over the first 7 days on ECMO support. The primary outcome was the rate of ICH during VV ECMO support.
Results
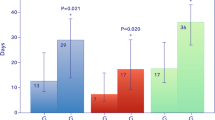
141 critically ill COVID-19 patients were included in the study. Patients with lower anticoagulation targets had consistently lower anti-Xa activity values over the first 7 ECMO days (p < 0.001). ICH incidence was lower in patients in the lower anti-Xa group: 4 (8%) vs 32 (34%) events. Accounting for death as a competing event, the adjusted subhazard ratio for the occurrence of ICH was 0.295 (97.5% CI 0.1–0.9, p = 0.044) for the lower anti-Xa compared to the higher anti-Xa group. 90-day ICU survival was higher in patients in the lower anti-Xa group, and ICH was the strongest risk factor associated with mortality (odds ratio [OR] 6.8 [CI 2.1–22.1], p = 0.001).
Conclusions
For COVID-19 patients on VV ECMO support anticoagulated with heparin, a lower anticoagulation target was associated with a significant reduction in ICH incidence and increased survival.
Similar content being viewed by others
Background
Despite an impressive gain of knowledge throughout the COVID-19 pandemic, the management of COVID-19 patients with acute respiratory distress syndrome (ARDS) remains challenging [1]. For these patients, venovenous extracorporeal membrane oxygenation (VV ECMO) can be a potential life-saving intervention facilitating lung protective ventilation [2, 3]. However, the use of ECMO is associated with serious complications such as an increased bleeding risk and hemotrauma [4]. In addition, patients with critical COVID-19 exhibit unique abnormalities in coagulation which can increase the risk of both bleeding and thrombotic events [31].
Aside from anticoagulation targets, management strategies of critically ill COVID-19 patients have been adapted during the course of the pandemic. In order to counteract profound inflammation, dexamethasone and tocilizumab have been increasingly used, with varying effects on patient outcomes [32,33,34]. From a pathophysiological viewpoint, it would be plausible that attenuated inflammation could decrease the risk of ICH, independent of anti-Xa levels. Indeed, we found decreased CRP and ferritin levels in the lower anti-Xa group, probably reflecting a more frequent use of anti-inflammatory drugs later in the pandemic. Interleukin 6 (IL-6) levels were slightly higher in the low anti-Xa group, in line with observations that IL-6 serum levels even can increase after administration of tocilizumab [35]. Taking dexamethasone and tocilizumab use into account in our analysis, the occurrence of ICH was still the main risk factor for mortality, whereas use of dexamethasone and tocilizumab was not associated with better patient survival. However, these results should be interpreted with caution, as we cannot rule out residual confounders affecting treatment decisions and inflammation. Of note, ECMO runtime was slightly longer and organ support tended to decrease in the low anti-Xa group, both probably affecting ICH risk. Thus, further large-scale studies specifically including patients requiring ECMO support should clarify effects of different anticoagulation strategies and the use of anti-inflammatory medication on global patient outcomes such as ICU length of stay, the need for mechanical ventilation, long-term neurologic sequelae of patients affected by bleeding, and mortality [31].
Our study has to account for limitations. We had a relatively small population of COVID-19 patients of VV ECMO, but overall mortality was similar to previous VV ECMO studies [10, 12, 36] and the consistency of the results across multiple centers adds generalizability to our findings. The retrospective nature of this study prevented us from inferring causality, and we cannot exclude the possibility of unmeasured confounders. Furthermore, our study did not analyze different viral mutants nor did it account for specific ICU patient management strategies such as ventilation, proning or sedation strategies. These all might have contributed to the lower observed overall mortality in the low anti-Xa cohort. The lower anti-Xa target was deployed later in the COVID-19 pandemic in response to the higher rates of ICH observed in earlier phases of the pandemic. Eligibility criteria for VV ECMO and practices for ICU and ventilator management during VV ECMO support evolved to some degree at the participating centers over the course of the pandemic, potentially contributing to higher survival rates in the lower anti-Xa group.
Conclusions
A less intense anticoagulation target (anti-Xa activity 0.15–0.3 U/mL) was associated with a decreased incidence of ICH and lower mortality in COVID-19 patients on VV ECMO support. Our results highlight the need for prospective studies to evaluate anticoagulation regimens in ARDS patients on ECMO support.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- anti-Xa:
-
Anti-factor Xa activity
- ARDS:
-
Acute respiratory distress syndrome
- CCT:
-
Cranial computed tomography
- ELSO:
-
Extracorporeal Life Support Organization
- ICH:
-
Intracerebral hemorrhage
- ICU:
-
Intensive care unit
- IL-6:
-
Interleukin 6
- RT-PCR:
-
Real-time reverse transcriptase-polymerase chain reaction
- SOFA score:
-
Sequential Organ Failure Assessment score
- UFH:
-
Unfractionated heparin
- VV ECMO:
-
Venovenous extracorporeal membrane oxygenation
References
COVID-19 Excess Mortality Collaborators (2022) Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 399:1513–1536
Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, Agerstrand C, Bartlett RH, Barrett N, Combes A, Lorusso R, Mueller T, Ogino MT, Peek G, Pellegrino V, Rabie AA, Salazar L, Schmidt M, Shekar K, MacLaren G, Brodie D, ELSO COVID-19 Working Group Members (2021) Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J 67:485–495
Shaefi S, Brenner SK, Gupta S, O’Gara BP, Krajewski ML, Charytan DM, Chaudhry S, Mirza SH, Peev V, Anderson M, Bansal A, Hayek SS, Srivastava A, Mathews KS, Johns TS, Leonberg-Yoo A, Green A, Arunthamakun J, Wille KM, Shaukat T, Singh H, Admon AJ, Semler MW, Hernán MA, Mueller AL, Wang W, Leaf DE, Investigators STOP-COVID (2021) Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med 47:208–221
Sniderman J, Monagle P, Annich GM, MacLaren G (2020) Hematologic concerns in extracorporeal membrane oxygenation. Res Pract Thromb Haemost 4:455–468
Liao D, Zhou F, Luo L, Xu M, Wang H, **a J, Gao Y, Cai L, Wang Z, Yin P, Wang Y, Tang L, Deng J, Mei H, Hu Y (2020) Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 7:e671–e678
Doyle AJ, Hunt BJ, Sanderson B, Zhang J, Mak SM, Benedetti G, Breen KA, Camporota L, Barrett NA, Retter A (2021) A comparison of thrombosis and hemorrhage rates in patients with severe respiratory failure due to coronavirus disease 2019 and influenza requiring extracorporeal membrane oxygenation. Crit Care Med 49:e663–e672
Nixon P, Butt W (2021) COVID-19: a new challenge for ECMO. Perfusion 36:573–574
Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18:1747–1751
Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, Zakhary B, Ramanathan K, Starr J, Akkanti B, Antonini MV, Ogino MT, Raman L, Barret N, Brodie D, Combes A, Lorusso R, MacLaren G, Müller T, Paden M, Pellegrino V, ELSO Guideline Working Group (2020) Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J 66:707–721
Seeliger B, Doebler M, Hofmaenner DA, Wendel-Garcia PD, Schuepbach RA, Schmidt JJ, Welte T, Hoeper MM, Gillmann HJ, Kuehn C, Ehrentraut SF, Schewe JC, Putensen C, Stahl K, Bode C, David S (2022) Intracranial hemorrhages on extracorporeal membrane oxygenation: differences between COVID-19 and other viral acute respiratory distress syndrome. Crit Care Med 50:e526–e538
Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, Lansac E, Sage E, Cholley B, Mégarbane B, Cronier P, Zarka J, Da Silva D, Besset S, Morichau-Beauchant T, Lacombat I, Mongardon N, Richard C, Duranteau J, Cerf C, Saiydoun G, Sonneville R, Chiche JD, Nataf P, Longrois D, Combes A, Leprince P, Paris ECMO-COVID-19 investigators (2021) Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med 9:851–862
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, EOLIA Trial Group, REVA, ECMONet (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378:1965–1975
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 22:707–710
McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM (2022) 2021 ELSO adult and pediatric anticoagulation guidelines. ASAIO J 68:303–310
ELSO guidelines for cardiopulmonary extracorporeal life support extracorporeal life support organization, version 1.4 August 2017 Ann Arbor, MI, USA. www.elso.org. Accessed 05 Jan 2023
Levy JH, Staudinger T, Steiner ME (2022) How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med 48:1076–1079
Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI (2020) Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7:e575–e582
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F, CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46:1089–1098
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, Griffin JH, Nugent D, Moon K, Morrissey JH (2022) Understanding COVID-19-associated coagulopathy. Nat Rev Immunol 22:639–649
Seeliger B, Döbler M, Friedrich R, Stahl K, Kühn C, Bauersachs J, Steinhagen F, Ehrentraut SF, Schewe JC, Putensen C, Welte T, Hoeper MM, Tiede A, David S, Bode C (2021) Comparison of anticoagulation strategies for veno-venous ECMO support in acute respiratory failure. Crit Care 24:701
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418
Godier A, Clausse D, Meslin S, Bazine M, Lang E, Huche F, Cholley B, Hamada SR (2021) Major bleeding complications in critically ill patients with COVID-19 pneumonia. J Thromb Thrombolysis 52:18–21
REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M, Reynolds HR, Kumar A, Turgeon AF, Kornblith LZ, Kahn SR, Marshall JC, Kim KS, Houston BL, Derde LPG, Cushman M, Tritschler T, Angus DC, Godoy LC, McQuilten Z, Kirwan BA, Farkouh ME, Brooks MM, Lewis RJ, Berry LR, Lorenzi E, Gordon AC, Ahuja T, Al-Beidh F, Annane D, Arabi YM, Aryal D, BaumannKreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Contreras A, Costantini TW, de Brouwer S, Detry MA, Duggal A, Džavík V, Effron MB, Eng HF, Escobedo J, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Froess JD, Fu Z, Galanaud JP, Galen BT, Gandotra S, Girard TD, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Haniffa R, Hegde SM, Hendrickson CM, Higgins AM, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Huang DT, Hudock K, Hunt BJ, Husain M, Hyzy RC, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski A, King AJ, Knudson MM, Kornblith AE, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Gallego Lima F, Linstrum K, Litton E, Lopez-Sendon J, Lother SA, Marten N, Saud Marinez A, Martinez M, Mateos Garcia E, Mavromichalis S, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nicolau JC, Nunez-Garcia B, Park JJ, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pompilio M, Quigley JG, Rosenson RS, Rost NS, Rowan K, Santos FO, Santos M, Santos MO, Satterwhite L, Saunders CT, Schreiber J, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Singhal AB, Slutsky AS, Solvason D, Stanworth SJ, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Widmer RJ, Wilson JG, Yuriditsky E, Zhong Y, Berry SM, McArthur CJ, Neal MD, Hochman JS, Webb SA, Zarychanski R (2021) Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med 385:777–789
von Stillfried S, Bülow RD, Röhrig R, Meybohm P, Boor P, German Registry of COVID-19 Autopsies (DeRegCOVID), DeRegCOVID Collaborators# (2022) Intracranial hemorrhage in COVID-19 patients during extracorporeal membrane oxygenation for acute respiratory failure: a nationwide register study report. Crit Care 26:83
Seeliger B, Wendel-Garcia PD, Stahl K, Bode C, David S (2022) It takes two to bleed: anticoagulation intensity and the host’s vascular susceptibility. Intensive Care Med 48:619–620
Rhoades R, Leong R, Kopenitz J, Thoma B, McDermott L, Dovidio J, Barletti S, Gong JZ, Massey HT, McKenzie SE, Rame JE, Al-Rawas N (2021) Coagulopathy monitoring and anticoagulation management in COVID-19 patients on ECMO: advantages of a heparin anti-Xa-based titration strategy. Thromb Res 203:1–4
Willems A, Roeleveld PP, Labarinas S, Cyrus JW, Muszynski JA, Nellis ME, Karam O (2021) Anti-Xa versus time-guided anticoagulation strategies in extracorporeal membrane oxygenation: a systematic review and meta-analysis. Perfusion 36:501–512
Mansour A, Flecher E, Schmidt M, Rozec B, Gouin-Thibault I, Esvan M, Fougerou C, Levy B, Porto A, Ross JT, Para M, Manganiello S, Lebreton G, Vincentelli A, Nesseler N, ECMOSARS Investigators (2022) Bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: a nationwide cohort study. Intensive Care Med 48:1039–1052
Raasveld SJ, Volleman C, Combes A, Broman LM, Taccone FS, Peters E, Ten Berg S, van den Brom CE, Thiele H, Lorusso R, Henriques JPS, Vlaar APJ (2022) Knowledge gaps and research priorities in adult veno-arterial extracorporeal membrane oxygenation: a sco** review. Intensive Care Med Exp 10:50
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2021) Dexamethasone in hospitalized patients with covid-19. N Engl J Med 38:693–704
RECOVERY Collaborative Group (2021) Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397:1637–1645
Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, Cameron ML, Garcia-Diaz J, Chávez V, Mekebeb-Reuter M, Lima de Menezes F, Shah R, González-Lara MF, Assman B, Freedman J, Mohan SV (2021) Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med 384:20–30
Hofmaenner DA, Wendel Garcia PD, Ganter CC, Brugger SD, Buehler PK, David S (2021) What every intensivist should know about tocilizumab. Crit Care 25:262
Herrmann J, Lotz C, Karagiannidis C, Weber-Carstens S, Kluge S, Putensen C, Wehrfritz A, Schmidt K, Ellerkmann RK, Oswald D, Lotz G, Zotzmann V, Moerer O, Kühn C, Kochanek M, Muellenbach R, Gaertner M, Fichtner F, Brettner F, Findeisen M, Heim M, Lahmer T, Rosenow F, Haake N, Lepper PM, Rosenberger P, Braune S, Kohls M, Heuschmann P, Meybohm P, German ECMO COVID Study Group (2022) Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit Care 26:190
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Consortia
Contributions
DAH, DF, LCW, EWG, SS, SD, BS and CB designed the study protocol. DAH, DF, LCW, EBK, AP, EWG and SS collected the data. PDWG and BS did the formal statistical analysis. All authors discussed and interpreted the findings. DAH wrote the initial draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed and data were acquired with approval of all responsible local ethics committees (Kantonale Ethikkommission Zürich, BASEC 2021-00825; Ethikkommission University Hospital Bonn number 196/21; BIDMC Committee on Clinical Investigation under IRB protocol 2017P000310) and shared through an executed Data Use Agreement.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Linear mixed effect model for the prediction of the anti-Xa activity.
Additional file 2: Table S2.
Competing risk regression model for intracranial hemorrhage treating death without intracranial hemorrhage as a competing event and study site as a frailty term.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hofmaenner, D.A., Furfaro, D., Wild, L.C. et al. Reduced anticoagulation strategy is associated with a lower incidence of intracerebral hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation. ICMx 11, 38 (2023). https://doi.org/10.1186/s40635-023-00525-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00525-3