Abstract
Modern medicine has been waging a war on cancer for nearly a century with no tangible end in sight. Cancer treatments have significantly progressed, but the need to increase specificity and decrease systemic toxicities remains. Early diagnosis holds a key to improving prognostic outlook and patient quality of life, and diagnostic tools are on the cusp of a technological revolution. Nanotechnology has steadily expanded into the reaches of cancer chemotherapy, radiotherapy, diagnostics, and imaging, demonstrating the capacity to augment each and advance patient care. Nanomaterials provide an abundance of versatility, functionality, and applications to engineer specifically targeted cancer medicine, accurate early-detection devices, robust imaging modalities, and enhanced radiotherapy adjuvants. This review provides insights into the current clinical and pre-clinical nanotechnological applications for cancer drug therapy, diagnostics, imaging, and radiation therapy.
Similar content being viewed by others
1 Introduction
Cancer devastates tens of millions of lives each year despite great advances in medicine and technology [1, 2]. Decades of research continuously reveal the ever-dynamic nature of the disease, and although treatment options have improved, severe side effects from harsh chemotherapies persist [3, 4]. Particularly, when aggressive cancers lie dormant then re-emerge, patients suffer when the need arises for more aggressive therapies [5,6,7]. One of the greatest challenges in finding a successful cancer treatment is the pervasive emergence of resistance mechanisms. Upon shutdown of initial oncogenic routes, resistance mechanisms are activated in parallel signaling pathways and re-route to allow for cancer to thrive [8, 9]. Heterogeneity can be found within different tumor cells, between patient tumors, amongst genetic mutations, and epigenetic patterns, all of which can limit responses to therapeutics, further allowing for drug resistance [10,11,12,13]. Clonal heterogeneity affects overall tumor biology and is known to drive metastasis and cancer progression [14]. Although new targets and therapies can advance cancer treatments, the dynamic nature of cancer finds a way to survive.
The strategy against cancer needs to shift from finding new therapies to improving existing therapies and diagnostics in innovative, effective, and plausible ways. Pain is experienced by 55% of patients undergoing cancer treatment and 66% of patients with advanced stage cancer [15]. Chemotherapies without distinct targeting mechanisms kill cancerous and noncancerous cells alike, therefore the systemic toxicity will continue to deteriorate patient quality of life [16, 17]. Furthermore, the benefits of early detection are clear. Cancer detected in early stages has a significantly higher 5-year survival rate, considerably lower overall cost to the patient, and typically less aggressive treatment course (Fig. 1) [18,19,20].
Late stage diagnoses for cancer results in significantly higher patient costs and decreased 5-year survival rates. The burden of cancer severely impacts patient quality of life with a majority experiencing pain directly from the disease and/or from treatment side effects. As the second leading cause of deaths worldwide, it is pertinent that new avenues are explored to improve cancer therapies and diagnostics
The solution may be found in nanotechnology: equip** existing therapies with better targeting capability, increasing localized drug efficacy, limiting systemic toxicity, improving diagnostic sensitivity, enhancing imaging, and refining radiation therapy [21,22,23,24]. Clinical translation of cancer nanomedicine dates back several decades, and the number of nano-based therapies and components for imaging, diagnostics, and radiation therapy in clinical use has steadily increased (Table 1) [25, 26]. For example, the CellSearch® system is the first FDA-approved diagnostic blood test which utilizes magnetic nanoparticles (NPs) targeting EpCAM and cell staining to identify circulating tumor cells [27]. Nano-based imaging contrast agents such as superparamagnetic iron oxide NPs (SPIONs) and Gadolinium (Gd)-based contrast agents enhance detection of tumor and imaging in vivo when using conventional scanning devices, such as magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT) [28].
Nanoformulations can counter resistance mechanisms by targeting multiple components with dual-drug loading, increasing specificity with triggered release, and utilizing physical modalities to eradicate cancerous cells [29, 30]. Nanoscale carriers can cross a tumor endothelium and passively accumulate in tumors owing to the leaky blood vessels and poor lymphatic drainage [31]. Furthermore, nanomaterials have unique physico-chemical properties which are employed in highly sensitive diagnostic tests, allowing for early detection of cancer and better patient prognosis [32, 33]. Cancer diagnostics are steadily moving away from invasive, complicated procedures to the direction of highly sensitive point-of-care liquid biopsies, where nanomaterials have demonstrated high utility for biomarker detection [34,35,36]. Certain properties also enable vast improvement of imaging techniques used for surgical guidance and tumor surveillance, enabling highly specific surgical resection and enhanced treatment monitoring [37]. Nanomaterials can function as radiosensitizers, creating highly specific and uniform radiation dosing to tumors while sparing healthy tissue [107, 108].
Cytotoxic and targeted therapies can select for drug resistance, therefore making complete eradication nearly impossible [109]. Drug resistance may develop through alterations in drug metabolism, changes in efflux/influx, hyper-activated repair pathways, signal transduction re-routing, and mutated drug targets [110, 111]. Methods for overcoming drug resistance include multiple therapeutics, combination chemoradiotherapy, and personalized medicine [112]. Co-administration of drugs with different molecular targets can help modulate cancer cell mutations and possibly halt the cancer adaptation process [113]. Effective combinations have been found where a drug can heighten or re-introduce sensitivity of the cancer cells to an existing therapy, and new combinatorial treatments are consistently being investigated in clinical trials. However, limitations exist for combination treatments largely due to different PK/PD properties and disjointed uptake of the complementary drugs, which reduces their efficacy and synergistic action. Co-delivery of anti-cancer therapies within a single nanocarrier can alleviate these issues and increase the therapeutic index [56, 114]. In 2017, the U.S. Food and Drug Administration (FDA) approved VYXEOS, a liposomal formulation of cytarabine and daunorubicin at a fixed 5:1 molar ratio, for the treatment of adults with newly diagnosed acute myeloid leukemia (AML) with myelodysplasia-related changes and therapy-related AML [115]. The synergistic molar ratio of daunorubicin and cytarabine has been shown to enhance the killing of leukemia cells in vitro and in murine models. In preclinical studies, VYXEOS liposomes were preferentially taken up by leukemia cells than by normal bone marrow cells in a murine model [227]. A single intracerebral injection of CRISPR-LNPs against PLK1 (sgPLK1-cLNPs) into aggressive orthotopic glioblastoma enabled up to ~ 70% gene editing in vivo, which caused tumor cell apoptosis, inhibited tumor growth by 50%, and improved survival by 30%. To reach disseminated tumors, cLNPs were also engineered for EGFR-targeted delivery. Intraperitoneal injections of EGFR-targeted sgPLK1-cLNPs caused their selective uptake into disseminated ovarian tumors, enabled up to ~ 80% gene editing in vivo, inhibited tumor growth, and increased survival by 80%. In another recent study, controlled release of CRISPR-Cas9 ribonucleoprotein (RNP) and codelivery with antitumor photosensitizer chlorin e6 (Ce6) was achieved using near-infrared (NIR)– and reducing agent–responsive NPs in a mouse tumor model [228]. Nitrilotriacetic acid–decorated micelles bound His-tagged Cas9 RNP, and lysosomal escape of NPs was triggered by NIR-induced reactive oxygen species (ROS) generation by Ce6 in tumor cells. Reduction of disulfide bond allowed cytoplasmic release of Cas9/single-guide RNA (sgRNA) targeting the antioxidant regulator Nrf2, enhancing tumor cell sensitivity to ROS, and demonstrating synergistic therapy in vivo. A plethora of exciting nanotechnologies exist that substantially improve cancer therapies, but there remain some obstacles for clinical translation such as scalability, homogeneity, and regulatory guidelines.
4 Cancer diagnostics on the nanoscale
As the saying goes, “an ounce of prevention is worth a pound of cure”; with regards to cancer treatments, it may be worth one metric ton of cure. Development of cancer pharmaceuticals is a costly endeavor, to say the least. The cost for develo** a successful drug can enter the billion-dollar realm, and the majority of drug candidates do not pass clinical trials [229]. Each year, the total oncology pipeline consists of hundreds of molecules in late-stage development, but only 50 new small molecule anti-cancer drugs were approved by the FDA from 2015–2020 [296]. Indocyanine green (ICG) is fluorescent dye used to identify the lymphatic channels and decipher which nodes to remove [297]. By doing so, patients avoid a complete lymphadenectomy, however disease must be thoroughly staged for accurate prognosis and determination of appropriate treatment approach. Several clinical studies are currently underway to investigate different nanomaterials as lymph node tracers such as fluorescent cRGDY-PEG-Cy5.5-C quantum dots (ClinicalTrials.gov Identifier: NCT02106598), carbon NPs (NCT03550001, NCT04482803), and silica NPs (NCT04167969).
Despite advancements in traditional imaging devices regarding both preoperative diagnostics and staging, there remains room for improvement regarding sensitivity, resolution, and intraoperative procedures. Progress continues for enhancing image-guided surgeries by incorporating specific targeting, optically-active materials, and nano-sized probes for alternative modes of imaging [298, 299]. Nano-enhanced imaging has potential to drastically improve early-stage detection of metastases and residual tumor cells to improve patient prognoses. Ferumoxtran-10, an ultrasmall superparamagnetic iron oxide (USPIO) particle has proven to be a valuable contrast agent for detecting lymph node metastases using a 1.5 Tesla or 3 Tesla MRI scanner in various types of cancer [300]. It is currently being studied in several clinical trials for SLN map**, including to improve the resolution and sensitivity of nano-MRI by using a 7 Tesla scanner, particularly for small lymph nodes (ClinicalTrials.gov Identifier: NCT03280277, NCT04300673, NCT03817307, NCT02857218, NCT04261777). A precision nano-enhanced approach for monitoring disease progression utilizes triggered aggregation in the TME (Target-Enabled in situ Ligand Assembly [TESLA]) [301]. The particles are built in situ at tumor sites from precursors containing specific moieties which can form larger NPs only after being cleaved by enzymes specific to cancer cell apoptosis. The NPs carry various image contrast agents for monitoring tumor therapy response to optimize effective dosing regimens, and TESLA is currently being investigated for rectal and breast cancer (ClinicalTrials.gov Identifier: NCT02751606).
The margin status of a tumor remains the main prognostic factor after surgical resection in HNSCC [302]. Margin sizes are used to determine adjuvant therapy or need for re-operation, and currently no technology is available in the operating room which reliably supports tumor excision in terms of margin status [303]. In fact, surgeons can only combine pre- operative imaging data with tactile and visual information during surgery for assessing tumor margins with limited accuracy. Near infrared (NIR) fluorescent optical contrast agents can be coupled to targeted compounds to create highly specific and well-resolved image-guided assessment of tumor margins [304]. Tracers utilize antibodies directed against VEGF-A (bevacizumab-IRDye800CW) for breast cancer or against EGFR, (cetuximab-IRDye800CW) for malignant glioma and pancreatic cancer (ClinicalTrials.gov Identifier: NCT01508572, NCT02855086, NCT02736578). First trials have shown that systemic administration of these compounds is safe and tumor specific. Clinical trials for the intraoperative assessment of tumor margins during surgical treatment of HNSCC and esophageal squamous cell carcinoma are currently underway using cetuximab-IRDye800CW (ClinicalTrials.gov Identifier: NCT03134846, NCT04161560).
4.3 The future of cancer diagnostics and imaging
As previously discussed, non-invasive, sensitive methods for cancer screening will be the key to clinically relevant diagnostics. Ultrasmall gold nanoclusters (AuNCs) have been found to make excellent probes for in vivo imaging because of their accumulation at tumor sites and efficient clearance via urine [305]. Multifunction protease nanosensors that react in the cancer cell microenvironment produce a colorimetric signal that could be monitored via urine. It was found in collected urine samples from colorectal cancer mouse models that tumor affected mice had a 13-fold increase in signal compared to healthy mice. Furthermore, novel imaging agents with better sensitivity and specificity can improve early detection during routine screening and help with tumor margin visualization during surgical resection. Recently, optical properties were investigated for multiple dyes and pigments used in tattoo inks, foods, drugs, and cosmetics already FDA approved [306]. Absorption, fluorescence, and Raman scattering properties were evaluated, and several exhibit a multitude of useful optical properties, outperforming some of the clinically approved imaging dyes on the market. The best performing optical inks (Green 8 and Orange 16) were formulated into liposomal NPs to assess their tumor targeting and optical imaging potential in mouse xenograft models of colorectal, cervical and lymphoma tumors. After intravenous injection, fluorescence imaging revealed significant localization of the new “optical ink” liposomal NPs in all three tumor models as opposed to their neighboring healthy tissues (p < 0.05). Nanoformulations of highly sensitive imaging contrast agents have potential to greatly improve cancer imaging, diagnosis, and surgical removal of tumor tissue.
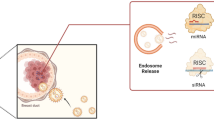
Nanotechnology has greatly impacted the realm of genetic sequencing through various nanopore-based systems, and subsequently, the realm of disease screening. The single molecule real time sequencing (SMRT) system is based on a single DNA polymerase within 60–100 nm cavities prepared by electron beam lithography on a thin aluminum 100 nm sheet deposited on a silica substrate [307]. This technique allows for optical monitoring of DNA sequence with use of fluorescent nucleotides added to the complement strand. Oxford Nanopore relies on passing a single DNA molecule through a nano-sized protein pore set within an electrically-resistant polymer membrane, where each DNA nucleotide base causes specific disruption in the current passing across the membrane [308]. Although both techniques present immense utility for omics data collection, circulating tumor DNA (ctDNA) analysis remains a challenge [309]. Recently, a new method using statistical analysis of the length of time for genetic code to unzip and blocking of the current has shown promise in identifying the precise position of genetic mutations [310]. This proof-of-concept study was demonstrated on oligonucleotides and is being further developed for liquid biopsies. Alternatively, targeted extracellular vesicle (EV) capture holds promise for liquid biopsy development since miRNA, mRNA, and proteins in/on EVs represent potential cancer biomarkers [311]. A high-throughput nano-biochip (HNCIB) for high-efficiency, targeted EV capture was recently developed using total internal reflective fluorescence microscopy for detection. HNCIB detected an up-regulated expression of programmed death-ligand 1 mRNA and protein and miR-21 in EVs derived from patients with lung adenocarcinoma compared to those from healthy donors. In addition to its high-throughput capabilities, it has low sample requirement and fast assay time. EV monitoring has further been useful for drug treatment monitoring effects, which was previously limited to invasive tissue biopsies and complex processes to analyze drug-target interactions. EV monitoring of small-molecular chemical occupancy and protein expression (ExoSCOPE) measures changes in drug occupancy and the composition of proteins present of in small volumes of blood to assess diseases status and success of targeted treatments [312]. It measures changes in drug occupancy and protein composition in molecular subpopulations of extracellular vesicles, and when used to monitor various targeted therapies, the ExoSCOPE revealed EV signatures that closely reflected cellular treatment efficacy. Using a small volume of blood, the ExoSCOPE accurately classified disease status and rapidly distinguished between targeted treatment outcomes, within 24 h after treatment initiation.
Theranostics aim to deliver point-of-care diagnosis and treatment with the same nanoformulation [313]. Theranostic agents can monitor the accumulation of nanomedicine compounds at the target site, visualize biodistribution, quantify triggered drug release, and assess therapeutic efficacy [320]. Absorptions of picosecond pulses of light by the SWCNTs creates photoacoustic induced cellular destruction without destroying the nearby environment allowing for continuous monitoring.
A and B Synthesis of boron-dipyrromethene compounds and subsequent assembly of nanocarriers functionalized with cell death-ligand 1 (PD-L1) monoclonal antibody. C and D Schematic of the BDP-I-N-anti-PD-L1-mediated phototoxicity and immune efficacy for tumor cells. Reprinted with permission, [319] https://doi.org/10.1021/acsnano.0c05317
5 Cancer radiation therapy
5.1 Current approaches to radiotherapy
Approximately 60% of cancer patients receive radiation treatment during the course of disease, depending on cancer type, stage, and time of diagnosis [321]. Radiation therapy (RT) is highly utilized to combat cancer because of its effectiveness in inducing DNA damage and subsequent cellular death, particularly in rapidly dividing cancer cells [322]. Although highly effective, radiotherapy is still not localized enough to avoid harmful effects on other parts of the body. Combination chemoradiotherapy is standard of care for many types of cancer, but further increases likelihood of systemic toxicity, however certain technological advancements in the past few decades have led to significant improvements [323]. 3D conformal radiation treatments, such as stereotactic (body) radiotherapy, intensity-modulated RT and improved imaging systems (i.e., image-guided RT), coupled with superior understanding of tumor biology have increased cancer RT survival rates from 30 to 80% [3]. Lastly, some cancers are known to be resistant to radiotherapy thus utilizing nanomaterials to enhance and hone specificity can greatly reduce toxicity of treatment [324].
5.2 Emerging nanotechnologies for RT in clinical applications
RT can benefit from nanotechnology enhancements since nanomaterials have specific properties conducive to atomic-level interactions with radiation and tumoral accumulation. High atomic number NPs have been shown to enhance Compton and photoelectric effects of conventional RT, and certain nanomaterials can utilize radiation-triggered drug release while others can serve as radiosensitizers [325, 326]. There are several clinical studies underway that utilize nanomaterials for enhancing RT further elucidated in this section (Table 4).
AGuIX is a nanoparticle composed of polysiloxane-based inorganic matrix bound to chelating agent DOTA (1,4,7,10-tetra-azacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid) covalently bound to the paramagnetic contrast enhancer Gd [327, 328]. Upon placement in a magnetic field, AGuIX produces a large magnetic moment and subsequently a large local magnetic field, which can enhance the relaxation rate of nearby protons, increasing MRI signal in tumor tissues where they have accumulated. The ultra-small NPs, less than 5 nm in diameter, allow for rapid renal clearance and reduced toxicity, and amplified radiation effects of AGuIX NPs were recently elucidated, attributed to the emission of low-energy photoelectrons and Auger electron interactions [329]. The preclinical study showed AGuIX NPs exacerbated radiation-induced DNA double-strand break damage and reduced DNA repair in the H1299 NSCLC cell line and is currently being tested in clinical trials (ClinicalTrials.gov Identifier: NCT04789486).
Certain NPs can combine radiation with localized PDT to induce tumor tissue destruction via ROS generation and radiosensitization, providing the benefit of physical ablation to deep tissue targets [330]. AuroLase therapy is a particle-directed photothermal therapy used with infrared-absorbing Auroshell NPs within tumor tissue to generate lethal doses of heat [331]. Auroshell particles consist of a gold metal shell surrounding a silica core, and a near-infrared (NIR)-tuned optical fiber can specifically deliver photonic laser energy which the NPs can absorb and create lethal photothermal lesions that are confined to the tumor tissue where Auroshells have accumulated [332]. In initial clinical studies, NP-mediated focal laser ablation was successful in 15/16 prostate cancer patients, and they are currently being investigated in an expanded clinical study (ClinicalTrials.gov Identifier: NCT04240639).
Normal X-ray radiation induces DNA damage through ROS generation after interaction with water molecules. NBTXR3 are hafnium oxide NPs engineered to increase energy deposit due to high electron density, thereby inducing greater oxidative stress within tumor cells and subsequent physical ablation [333]. Soft tissue sarcomas of limbs or trunk enable direct injection of NPs into the tumor, where the radiotherapy enhancement can be localized to cancerous tissue, but locally advanced soft tissue sarcomas (high risk that are typically unresectable) often requires pre-operative radiotherapy, making ideal cancer types for testing NBTXR3 [334]. In a recent phase 2/3 trial (ClinicalTrials.gov Identifier: NCT02379845), the rate of pathologic complete response (< 5% remaining viable tumor cells) was achieved in twice as many patients in test arm as in the control arm (16 vs 8%; P = 0.044), and the NPs were well tolerated. NBTXR3 is currently being evaluated in 8 clinical studies on various cancers (ClinicalTrials.gov Identifiers: NCT01946867, NCT04505267, NCT03589339, NCT04484909, NCT04615013, NCT04862455, NCT04834349, NCT04892173). Radiation-induced liver disease (RILD) or radiation hepatitis is a sub-acute form of liver injury due to radiation [335]. It is one of the most severe side effects of radiation which prevents radiation dose escalation and re-irradiation for hepatobiliary or upper gastrointestinal malignancies. Hepatic cirrhosis in patients with hepatocellular carcinomas (HCC), or chemotherapy-induced hepatic atrophy or hepatosteatosis in patients with liver metastases can be associated with high risk of RILD after stereotactic body radiotherapy (SBRT) [336, 337]. However, hepatotoxicity can be greatly reduced by switching to MRI-guided radiotherapy with SPION on 1.5 Tesla MR-Linac as opposed to nuclear medicine [338]. MRI-SPION radiotherapy is expected to facilitate detection and maximize avoidance of residual, functionally-active hepatic parenchyma from over-the-threshold irradiation, thus increasing safety of liver stereotactic body radiotherapy in patients with pre-existing liver conditions (ClinicalTrials.gov Identifier: NCT04682847).
5.3 Nanomaterials to improve and augment RT
Drug resistance and cancer heterogeneity can be addressed by physical destruction of tumor cells through RT, but there is room for improvement with respect to specificity and enhanced efficacy. Previous research has shown that radiotherapy can be utilized to activate the immune system by inducing immunogenic cell death (ICD), an immune response against the antigens of dead or dying tumor cells [339]. ICD-associated damage via ROS production could possibly promote the activation and migration of dendritic cells to prime T cells for systemic anti-tumor immune responses [340, 341]. However, radiation-stimulated immune responses have shown limited efficacy, particularly when tumors exhibit low X-ray absorption and energy deposition capacities [342, 343]. Disjoint oxygen supply and demand within tumors result in hypoxic areas with high levels of hydrogen peroxide, which induce adaptive antioxidant mechanisms [344, 345]. Subsequently, high concentrations of reducing substances, such as glutathione, quench •OH generated by RT, ultimately reducing its efficacy [346]. One solution to amplify RT mediated oxidative stress to induce ICD for antitumor immunity activation was developed via a novel radiosensitizer that incorporates nanoscale coordination polymers (NCPs) based on Gd3+ and 5′-Guanosine monophosphate (5′-GMP) via supramolecular self-assembly (Fig. 9) [347]. Hemin (PANHEMATIN®) with peroxidase-mimic catalytic activity was incorporated into the Gd3+/5′-GMP NCPs (Gd-NCPs) to form Hemin@ Gd3+/5′-GMP NCPs (H@Gd-NCPs). Furthermore, presence of metal element Gd, H@Gd-NCPs can act as an MRI contrast agent, adding to its utility for clinical use. The H@Gd-NCPs effectively enhance X-ray absorption and produce more ROS, especially hydroxyl radicals within tumor tissues. The encapsulated hemin can enhance peroxidase-like properties to utilize overexpressed hydrogen peroxide in TME to deplete GSH. Combination of ROS enhancement and GSH depletion amplifies irradiation-mediated oxidative stress and induce ICD. The antitumor immunity activated by H@Gd-NCPs can further be strengthened by immune checkpoint blockade therapy against primary, distant, and metastatic tumors. Another approach to address hypoxic environmental impact on RT is to utilize nitric oxide (NO) prodrugs, shown to be efficient radiosensitizers as cell respiration inhibitors, and co-delivery with exogenous oxygen resources [348, 349]. Recent work has shown potential solution to this obstacle through a hybrid semiconducting organosilica-based O2 nanoeconomizer which in an acidic tumor environment releases NO and, via mild photothermal treatment, releases O2 resulting in enhanced efficacy of radiotherapy in vitro and in vivo [350]. A semiconducting polymer brush (SPB) framework has an electron donor and acceptor backbone, providing NIR II fluorescence, photoacoustic contrast, and photothermal conversion for theranostic application. It can be tuned for mild hyperthermia with tumor oxygenation improvement to boost RT, or higher temperature physical ablation. A hybridized fluorocarbon (FC) chain provides ease for oxygen loading and photothermally-controlled release, and in situ polymerization of PEG and alkyl chains improves biocompatibility and loading/retention of NO prodrugs. This novel nanoplatform (pHPFON-NO/O2) demonstrates tunable, pH-activated NO release and radiation-activated O2 delivery for enhanced radiosenstivity. An alternative to photon irradiation is the use of fast ion beams (proton therapy and hadron therapy [70–400 MeV amu−1]) to treat solid tumors [351]. Since ion irradiation has more specific tumor targeting, they are generally used for tumors in highly sensitive tissues such as eyes and brain, pediatric cancers, and/or radioresistant tumors [352]. However, one significant drawback remains as the damage sustained to healthy tissue in front of the tumor, so radiosensitizers can amplify the radiation effects within the tumor area while lowering dosage to the healthy tissue. [353] A Gd-chelated polysiloxane matrix based-nanoparticle was recently engineered to increase dose effect through generation of a high number of radicals via direct or indirect interaction of high-energy particles with Gd [354].The efficiency of AGuIX NPs to amplify the effects of medical protons was demonstrated using a 150 MeV proton beam under two irradiation conditions mimicking the entrance (0.44 keV µm−1) and the end (3.6 keV µm−1) of the proton track on plasmid pBR322, and is currently under clinical investigation in France [327].
A Preparation of a novel radiosensitizer that incorporates nanoscale coordination polymers (NCPs) based on gadolinium (Gd3+) and 5′-Guanosine monophosphate (5′-GMP) via supramolecular self-assembly. B Mechanism of radiosensitization via amplification of radiotherapy-mediated oxidative stress. Dendritic cells (DCs), glutathione (GSH), oxidized glutathione (GSSG), hydroxyl radicals (•OH), calreticulin (CRT), high mobility group protein B1 (HMGB1), adenosine triphosphate (ATP). Reprinted with permission, [347] https://doi.org/10.1038/s41467-020-20243-8
Hyperthermia (localized heat to kill cells) is a promising method for elimination of cancerous tissue, and certain nanomaterials have been shown to enhance hyperthermal effects [355, 356]. Uniform and selective hyperthermia can be achieved using nanomaterials with a high-absorption cross-section, which can convert an external energy source into heat [357, 358]. Dynamic nanomaterials can achieve maximum therapeutic effects through multi-modal cancer treatments. Multi-modal treatments pose the possibility of eliminating cancer via physical activation such as photothermal, photodynamic, radiation, and magnetic. Specifically, gold and carbon nanomaterials have been extensively used to induce hyperthermal effects upon near-infrared light (NIR) irradiation [248, 359]. They can further be utilized for triggered drug release with therapeutics tethered to the surface or encapsulated within, and payload can release upon change in temperature or irradiation [360, 361]. This approach can incorporate a combinatorial approach by using therapeutic agents in conjunction with an external trigger to localize treatment and/or destroy cancerous cells with physical ablation [362]. Recently, activatable polymeric pro-nanoagonist (APNA) triggers tumor ablation by the photothermal effect and induces immunogenic cell death and is activated by second near-infrared (NIR-II) light which enables deep-tissue penetration and represents a strategy for future RT [363]. APNA is constructed from covalent conjugation of toll-like receptor type 7 and 8 agonist (Resiquimod: R848) onto a NIR-II semiconducting transducer through a labile thermo-responsive linker. Upon NIR-II photoirradiation, APNA mediates photothermal effect, triggers tumor ablation, immunogenic cell death, and initiates the cleavage of thermolabile linker to liberate caged agonist for in-situ immune activation in deep solid tumor (8 mm). Cancer stem cells are particularly concerning due to their resistance for anticancer drugs, thus alternative methods are necessary [364]. A novel approach to utilizing nanomaterials with radiation is photothermal control of heat-sensitive TRPV1 or TRPV2 ion channels to regulate cell stemness [365]. This recent study demonstrates NIR-photoactive nanocarbon complexes can stimulate TRPV2 overexpression in cancer cells, disrupting intracellular Ca2+ regulation, suppressing Wnt/β-catenin signaling, which resulted in the destruction of cancer cells and inhibition of stemness in both in vitro and in vivo models.
Although photothermal agents (PTAs) have shown promising results in clinical studies, rapid degradation of PTA limits the photothermal stability required for efficacious treatment yet those with high photothermal stability degrade slowly thus have greater safety concerns [366, 367]. Currently, there are few PTAs with high photothermal stability and rapid degradation. Recently, it was shown that the inherent Cu2+-capturing ability of black phosphorus (BP) can accelerate the degradation of BP, while also enhancing photothermal stability [368]. The incorporation of Cu2+ into BP@Cu nanostructures further enables chemodynamic therapy-enhanced PTT. Moreover, by employing 64Cu2+, PET imaging can be achieved for in vivo real-time and quantitative tracking.
6 Perspectives and conclusion
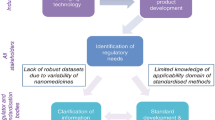
Nanomaterials are highly versatile, adaptable, and have many advantages that can improve cancer treatments and diagnostics (Fig. 10). However, factors such as production cost, scalability, safety, and complexity of nanoformulations must be considered and weighed against the potential benefits. As complexity of design and materials increases, so do costs, manufacturing criteria, and testing parameters [144]. Some nanomedicines may present a clear clinical benefit over conventional formulation, but if cost and production requirements are unattainable, clinical translation may never be realized. An increasingly important factor for commercial production is the environmental impact, not only of the nanomaterials themselves, but manufacturing by-products and energy costs [369]. In addition, there is a shadow of uncharted territory for FDA approval that many nanomedicines may face. The FDA has 3 product areas based on whether the product has a chemical mode of action (drug), a mechanical mode of action (device), or a biological source (biologic), and certain nanoformulations can span all 3 areas, categorizing them as a combination product. With rapidly advancing technologies for nanomedicine, there seems to be a need for more consistent and robust guidelines to evaluate clinical trials for nanomaterials. In 2006, the FDA Nanotechnology Task Force was created to address the regulatory deficiencies regarding nanoformulated medicines and devices, but determined that no new regulations were warranted [370]. The FDA published two documents regarding nanotechnology application and status, risk-based framework, specific requirements for conduct of nonclinical and clinical trials, manufacturing quality and controls, and environmental considerations [371, 372]. However, considering the pace and magnitude of nanotechnology research, a 15-year-old guidance is now exceedingly outdated. Although breakthrough status and subsequent accelerated approval can be achieved for certain drugs/devices/biologics, the gap in cohesive regulation for nanotechnology remains unclosed. Without comprehensive, updated evaluation and policy regarding nanotechnology in medicine and devices, the cost vs. benefit analysis will be unclear, and possibly a roadblock for critical research.
Conventional cancer therapies, diagnostics, radiation treatments, and imaging can be significantly improved through nanotechnological applications. Nanotechnologies are emerging in all fields at an increasing rate, and future applications hold great promise to significantly improve patient prognoses and quality of life
Cost vs. benefit analysis of nanomedicine poses many questions even without the issue of unclear regulatory guidelines. Nanomedicine can have much higher manufacturing costs than conventional drugs, depending upon formulation and complexity, however it is not as simple as comparing apples to apples [144]. Quality of life is typically only addressed for the duration of clinical trials; a recent study found that in only 5 of 149 studies (3.4%), quality of life was assessed until death, with only 1 out of 74 studies on metastatic or incurable cancers, and it is often not a co-primary endpoint [373]. Of course, quality of life may not be assigned a price, but certain quantifiable metrics may be applicable to evaluating the worth of nano-focused research and development. A 2011 survey found that one third of cancer survivors experienced limitations in their ability to perform usual daily activities outside of work, 25.1% felt cancer interfered with physical work tasks, and 24.7% overall felt less productive at work [374]. As previously noted, nanoformulations are often engineered to increase specificity, efficacy, and guard against drug resistance, thus patient quality of life is an important metric to evaluate for a prolonged period. There are many intricacies and considerations in determining the viability of drug products that go beyond merely cost of development vs. clinical outcome, and nanomedicine certainly adds to the equation.
Accessibility of information is at an apex, available at the touch of a button, which has greatly accelerated the advancement of scientific research. While extremely beneficial, it has also created a need for hierarchal and centralized organization as the scientific “toolbox” is flooded with data and new techniques. A search for the words “nano cancer” within only Nature publications for the year 2019 yielded 932 research articles, with Nature publications known to critically evaluate and publish highly impactful research. A recent study from MIT has constructed a machine learning framework to indicate “impactful” research that is overlooked by current metrics, opening the door to a possibility of machine-assisted direction of research [375]. While many in the scientific community were critical of the study and its implications, the idea of utilizing artificial intelligence to guide basic research has great potential. A multitude of parameters can be used to determine likelihood of clinical translation for nanotechnologies, as well as cross-reference with existing and emerging research to optimize formulation and strategy. Nanomedicine can greatly benefit from machine learning applications from analysis of patient tumor profiles and drug response to nanoparticle design, determining optimum material according to drug target, mechanistic attributes, and individualized prognoses [376, 377]. Machine learning was recently shown to estimate the cellular internalization of NPs based on their surface design, along with a machine learning-based model to sense breast cancer cells via internalization of eight differently functionalized carbon NPs (CNPs) [378]. The model accurately predicted the internalization of CNPs based on their structural features. NP cellular internalization were evaluated using different endocytic pathways, and artificial intelligence was then utilized to rank specific NP properties for optimum design. Furthermore, patient-specific cancer profiles were determined with machine learning techniques and cellular internalization profiles, demonstrating an efficient platform to render distinct fingerprints for individual cancer cell types. Neural networks are also demonstrating their use for diagnostics as well, from evaluating omics data to tumor imaging, and even optimizing radiotherapy [379,380,381,382].
The future of nanomedicine is certainly auspicious, with highly developed technologies improving treatments and diagnostics, and machine learning applications augmenting to save significant time and resources. There are multitudes of clinical and preclinical studies demonstrating the benefits of nanotechnology in cancer treatment, imaging, and diagnostics, but it is critical that these advances are clinically translatable. One key component in improving cancer patient outcome clearly lies in early detection methods. As previously discussed, early-stage cancers are generally much easier to treat, and early detection drastically improves 5-year survival rates and lowers patient cost. However, it is critical that diagnostic screenings are extremely accurate, otherwise misdiagnoses and overtreatments overshadow the benefits of early detection. Nanotechnology for cancer diagnostics, chemo- and radiotherapies stands to gain huge ground in the near future, creating a highly manageable cancer landscape for patients and oncologists. Although the dynamic nature of cancer refuses to yield, innovation continues to progress, and convergence of multiple technologies has promise to prevail.
Availability of data and materials
Not applicable.
Abbreviations
- PK/PD:
-
Pharmacokinetics/pharmacodynamics
- ADC:
-
Antibody drug conjugate
- AML:
-
Acute myeloid leukemia
- APC:
-
Antigen presenting cells
- APNA:
-
Activatable polymeric pro-nanoagonist
- ATO:
-
Arsenic trioxide
- BP:
-
Black phosphorus
- CAF:
-
Cancer-associated fibroblast
- CNP:
-
Carbon nanoparticle
- CPI:
-
Checkpoint-inhibitor
- CPT:
-
Camptothecin
- CT:
-
Computed tomography
- CTC:
-
Circulating tumor cell
- DCs:
-
Dendritic cells
- DOX:
-
Doxorubicin
- DRP:
-
Drug Response Prediction
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EMA:
-
European Medicines Agency
- EMT:
-
Epithelial-mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- EPR:
-
Enhanced permeation and retention
- EV:
-
Extracellular vesicle
- FAP:
-
Fibroblast activation protein
- FDA:
-
U.S. Food and Drug Administration
- HCC:
-
Hepatocellular carcinoma
- HLA:
-
Human leukocyte antigen
- HNCIB:
-
High-throughput nano-biochip
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- ICD:
-
Immunogenic cell death
- ICG:
-
Indocyanine green
- iNKT:
-
Invariant natural killer T
- LN:
-
Lymph node
- LNP:
-
Lipid nanoparticle
- mABs:
-
Monoclonal antibodies
- MRD:
-
Minimal residual disease
- MRI:
-
Magnetic resonance imaging
- MSN:
-
Mesoporous silica NPs
- NIR:
-
Near-infrared
- NIR-II:
-
Second near-infrared
- NO:
-
Nitric oxide
- NP:
-
Nanoparticle
- NY-ESO-1:
-
New York esophageal squamous cell carcinoma 1
- OMV:
-
Outer membrane vesicle
- PAA:
-
Polyacrylic acid
- PLGA:
-
Poly(lacto-co-glycolic acid)
- PLK1:
-
Polo-like kinase 1
- PTA:
-
Photothermal agent
- PTX:
-
Paclitaxel
- RILD:
-
Radiation-induced liver disease
- RNP:
-
Ribonucleoprotein
- RT:
-
Radiation therapy
- SBRT:
-
Stereotactic body radiotherapy
- sgRNA:
-
Single-guide RNA
- SIG:
-
Signature
- SLN:
-
Sentinel lymph node
- SMRT:
-
Single molecule real time
- SNALP:
-
Stable nucleic acid lipid particles
- SPION:
-
Superparamagnetic iron oxide nanoparticle
- SSR:
-
Somatostatin receptors
- SWCNT:
-
Single-walled carbon nanotube
- TAA:
-
Tumor associated antigen
- TCR:
-
T cell receptors
- TESLA:
-
Target-Enabled in situ Ligand Assembly
- TKI:
-
Tyrosine kinase inhibitor
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple negative breast cancer
- Topo-1:
-
Topoisomerase 1
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- TSA:
-
Tumor specific antigen
- USPIO:
-
Ultrasmall superparamagnetic iron oxide
- VEGF:
-
Vascular endothelial growth factor
- VOC:
-
Volatile organic compounds
References
M. Kabir, M. Rahman, R. Akter, T. Behl, D. Kaushik, V. Mittal, P. Pandey, M.F. Akhtar, A. Saleem, G.M. Albadrani, Biomolecules 11, 392 (2021)
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, CA Cancer J. Clin. 68, 394 (2018)
H.H. Chen, M.T. Kuo, Oncotarget 8, 62742 (2017)
V. Schirrmacher, Int. J. Oncol. 54, 407 (2019)
P. Khosravi-Shahi, L. Cabezón-Gutiérrez, S. Custodio-Cabello, Asia Pac. J. Clin. Oncol. 14, 32 (2018)
R. R. Aggarwal, F. Y. Feng, E. J. Small, Oncology 31, 467 (2017)
X.-L. Gao, M. Zhang, Y.-L. Tang, X.-H. Liang, Onco Targets Ther. 10, 5219 (2017)
N. Vasan, J. Baselga, D.M. Hyman, Nature 575, 299 (2019)
J. Rueff, A.S. Rodrigues, Cancer Drug Resistance (Springer, 2016), p. 1
X. Sun, J. Bao, Y. Shao, Sci. Rep. 6, 1 (2016)
M. Guo, Y. Peng, A. Gao, C. Du, J.G. Herman, Biomark. Res. 7, 1 (2019)
K. Hinohara, K. Polyak, Trends Cell Biol. 29, 569 (2019)
D.R. Caswell, C. Swanton, BMC Med. 15, 1 (2017)
M. Janiszewska, D.P. Tabassum, Z. Castaño, S. Cristea, K.N. Yamamoto, N.L. Kingston, K.C. Murphy, S. Shu, N.W. Harper, C.G. Del Alcazar, Nat. Cell Biol. 21, 879 (2019)
S. Iwase, T. Kawaguchi, A. Tokoro, K. Yamada, Y. Kanai, Y. Matsuda, Y. Kashiwaya, K. Okuma, S. Inada, K. Ariyoshi, PLoS ONE 10, e0134022 (2015)
H. Namazi, V.V. Kulish, A. Wong, Sci. Rep. 5, 1 (2015)
D. Lorusso, E. Bria, A. Costantini, M. Di Maio, G. Rosti, A. Mancuso, Eur. J. Cancer Care 26, e12618 (2017)
B. Moghimi-Dehkordi, A. Safaee, World J. Gastrointest. Oncol. 4, 71 (2012)
H.G. Welch, L.M. Schwartz, S. Woloshin, JAMA 283, 2975 (2000)
H. Blumen, K. Fitch, V. Polkus, Am. Health Drug Benefits 9, 23 (2016)
P. Falagan-Lotsch, E.M. Grzincic, C.J. Murphy, Bioconjug. Chem. 28, 135 (2017)
G. Caracciolo, H. Vali, A. Moore, M. Mahmoudi, Nano Today 27, 6 (2019)
S. Goel, D. Ni, W. Cai, ACS Nano 11, 5233 (2017)
J. Shi, P.W. Kantoff, R. Wooster, O.C. Farokhzad, Nat. Rev. Cancer 17, 20 (2017)
L.-P. Wu, D. Wang, Z. Li, Mater. Sci. Eng. C 106, 110302 (2020)
A. Wicki, D. Witzigmann, V. Balasubramanian, J. Huwyler, J. Control. Release 200, 138 (2015)
A. Truini, A. Alama, M.G. Dal Bello, S. Coco, I. Vanni, E. Rijavec, C. Genova, G. Barletta, F. Biello, F. Grossi, Front. Oncol. 4, 242 (2014)
D. L. Miyasato, A. W. Mohamed, C. Zavaleta, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. e1721 (2021)
J. Chen, Z. Jiang, W. Xu, T. Sun, X. Zhuang, J. Ding, X. Chen, Nano Lett. 20, 6191 (2020)
J.A. Kemp, M.S. Shim, C.Y. Heo, Y.J. Kwon, Adv. Drug Del. Rev. 98, 3 (2016)
J.L. Perry, K.G. Reuter, J.C. Luft, C.V. Pecot, W. Zamboni, J.M. DeSimone, Nano Lett. 17, 2879 (2017)
R. De La Rica, D. Aili, M.M. Stevens, Adv. Drug Del. Rev. 64, 967 (2012)
K. Niemirowicz, K. Markiewicz, A. Wilczewska, H. Car, Adv. Med. Sci. 57, 196 (2012)
J. Wang, K.M. Koo, Y. Wang, M. Trau, Adv. 6, 1900730 (2019)
N. Soda, B.H. Rehm, P. Sonar, N.-T. Nguyen, M.J. Shiddiky, J. Mater. Chem. B 7, 6670 (2019)
D.P. Kalogianni, Nano Converg. 8, 1 (2021)
R.M. Davis, J.L. Campbell, S. Burkitt, Z. Qiu, S. Kang, M. Mehraein, D. Miyasato, H. Salinas, J.T. Liu, C. Zavaleta, Nanomaterials 8, 953 (2018)
J. **e, L. Gong, S. Zhu, Y. Yong, Z. Gu, Y. Zhao, Adv. Mater. 31, 1802244 (2019)
A.J. Mieszawska, W.J.M. Mulder, Z.A. Fayad, D.P. Cormode, Mol. Pharm. 10, 831 (2013)
X. Chen, J. Gole, A. Gore, Q. He, M. Lu, J. Min, Z. Yuan, X. Yang, Y. Jiang, T. Zhang, Nat. Commun. 11, 1 (2020)
T.R. Rebbeck, K. Burns-White, A.T. Chan, K. Emmons, M. Freedman, D.J. Hunter, P. Kraft, F. Laden, L. Mucci, G. Parmigiani, Cancer Discov. 8, 803 (2018)
L. Salvioni, M.A. Rizzuto, J.A. Bertolini, L. Pandolfi, M. Colombo, D. Prosperi, Cancers (Basel) 11, 1855 (2019)
S. Tran, P.-J. DeGiovanni, B. Piel, P. Rai, Clin. Transl. Med. 6, 1 (2017)
E. Beltrán-Gracia, A. López-Camacho, I. Higuera-Ciapara, J.B. Velázquez-Fernández, A.A. Vallejo-Cardona, Cancer Nanotechnol. 10, 11 (2019)
V. Torchilin, Adv. Drug Del. Rev. 63, 131 (2011)
M. Izci, C. Maksoudian, B.B. Manshian, S.J. Soenen, Chem. Rev. 121, 1746 (2021)
J. Fang, W. Islam, H. Maeda, Adv. Drug Del. Rev. 157, 142 (2020)
H. Kang, S. Rho, W.R. Stiles, S. Hu, Y. Baek, D.W. Hwang, S. Kashiwagi, M.S. Kim, H.S. Choi, Adv. Healthc. Mater. 9, 1901223 (2020)
C.D. Walkey, J.B. Olsen, H. Guo, A. Emili, W.C. Chan, J. Am. Chem. Soc. 134, 2139 (2012)
G. Fullstone, J. Wood, M. Holcombe, G. Battaglia, Sci. Rep. 5, 1 (2015)
Y.Y. Chen, A.M. Syed, P. MacMillan, J.V. Rocheleau, W.C. Chan, Adv. Mater. 32, 1906274 (2020)
H.H. Gustafson, D. Holt-Casper, D.W. Grainger, H. Ghandehari, Nano Today 10, 487 (2015)
C.T. Curley, B.P. Mead, K. Negron, N. Kim, W.J. Garrison, G.W. Miller, K.M. Kingsmore, E.A. Thim, J. Song, J.M. Munson, Sci. Adv. 6, eaay1344 (2020)
M. Bartneck, H.A. Keul, G. Zwadlo-Klarwasser, J. Groll, Nano Lett. 10, 59 (2010)
J.Y. Yhee, S. Jeon, H.Y. Yoon, M.K. Shim, H. Ko, J. Min, J.H. Na, H. Chang, H. Han, J.-H. Kim, M. Suh, H. Lee, J.H. Park, K. Kim, I.C. Kwon, J. Control. Release 267, 223 (2017)
R. van der Meel, E. Sulheim, Y. Shi, F. Kiessling, W.J. Mulder, T. Lammers, Nat. Nanotechnol. 14, 1007 (2019)
J. Feng, M. Xu, J. Wang, S. Zhou, Y. Liu, S. Liu, Y. Huang, Y. Chen, L. Chen, Q. Song, J. Gong, H. Lu, X. Gao, J. Chen, Biomaterials 241, 119907 (2020)
C. Wang, Y. Yu, M. Irfan, B. Xu, J. Li, L. Zhang, Z. Qin, C. Yu, H. Liu, X. Su, Small 16, 2002578 (2020)
N. Rabiee, M. Bagherzadeh, A. M. Ghadiri, Y. Fatahi, A. Aldhaher, P. Makvandi, R. Dinarvand, M. Jouyandeh, M. R. Saeb, M. Mozafari, ACS Appl. BioMater. 13, 5336 (2021)
P.K. Gupta, R. Gahtori, K. Govarthanan, V. Sharma, S. Pappuru, S. Pandit, A.S. Mathuriya, S. Dholpuria, D.K. Bishi, Mater. Sci. Eng. C 127, 112198 (2021)
I. Pontón, A. Martí del Rio, M. Gómez Gómez, D. Sánchez-García, Nanomaterials 10, 2466 (2020)
M. Ye, Y. Han, J. Tang, Y. Piao, X. Liu, Z. Zhou, J. Gao, J. Rao, Y. Shen, Adv. Mater. 29, 1702342 (2017)
M.D. Norris, K. Seidel, A. Kirschning, Adv. Ther. 2, 1800092 (2019)
J.D. Yoo, S.M. Bae, J. Seo, I.S. Jeon, S.M.P. Vadevoo, S.-Y. Kim, I.-S. Kim, B. Lee, S. Kim, Sci. Rep. 10, 19997 (2020)
S. Hossen, M.K. Hossain, M.K. Basher, M.N.H. Mia, M.T. Rahman, M.J. Uddin, J. Adv. Res. 15, 1 (2019)
J.D. Bargh, A. Isidro-Llobet, J.S. Parker, D.R. Spring, Chem. Soc. Rev. 48, 4361 (2019)
R. Qi, Y. Wang, P.M. Bruno, H. **ao, Y. Yu, T. Li, S. Lauffer, W. Wei, Q. Chen, X. Kang, Nat. Commun. 8, 1 (2017)
R.-Y. Guo, H.-M. Wang, X. Dong, Y. Hu, J. Li, Y. Zang, X. Li, ACS Appl. Bio Mater. 4, 2058 (2021)
T. Cheng, Y. Zhang, J. Liu, Y. Ding, H. Ou, F. Huang, Y. An, Y. Liu, J. Liu, L. Shi, A.C.S. Appl, Mater. Interfaces 10, 5296 (2018)
L. Rao, J.-H. Xu, B. Cai, H. Liu, M. Li, Y. Jia, L. **ao, S.-S. Guo, W. Liu, X.-Z. Zhao, Nanotechnology 27, 085106 (2016)
P. Navya, A. Kaphle, S. Srinivas, S.K. Bhargava, V.M. Rotello, H.K. Daima, Nano Converg. 6, 1 (2019)
S.-J. Tseng, I.M. Kempson, K.-Y. Huang, H.-J. Li, Y.-C. Fa, Y.-C. Ho, Z.-X. Liao, P.-C. Yang, ACS Nano 12, 9894 (2018)
M. Ovais, S. Mukherjee, A. Pramanik, D. Das, A. Mukherjee, A. Raza, C. Chen, Adv. Mater. 32, 2000055 (2020)
X. Liu, X. Wu, Y. **ng, Y. Zhang, X. Zhang, Q. Pu, M. Wu, J.X. Zhao, ACS Appl. Bio Mater. 3, 2577 (2020)
J.-M. Shen, T. Yin, X.-Z. Tian, F.-Y. Gao, S. Xu, A.C.S. Appl, Mater. Interfaces 5, 7014 (2013)
N. Kamaly, B. Yameen, J. Wu, O.C. Farokhzad, Chem. Rev. 116, 2602 (2016)
J. Kolosnjaj-Tabi, L. Gibot, I. Fourquaux, M. Golzio, M.-P. Rols, Adv. Drug Del. Rev. 138, 56 (2019)
L.L. Israel, A. Galstyan, E. Holler, J.Y. Ljubimova, J. Control. Release 320, 45 (2020)
Y. Meng, D. Zhang, X. Chen, Z. Dai, X. Yao, P. Cui, D. Yu, G. Zhang, X. Zheng, ACS Appl. Nano Mater. 3, 4494 (2020)
B. Sun, R. Chang, S. Cao, C. Yuan, L. Zhao, H. Yang, J. Li, X. Yan, J.C. van Hest, Angew. Chem. Int. Ed. 59, 20582 (2020)
J. Yao, M. Yang, Y. Duan, Chem. Rev. 114, 6130 (2014)
X. Chi, D. Huang, Z. Zhao, Z. Zhou, Z. Yin, J. Gao, Biomaterials 33, 189 (2012)
Y. Zeng, H. Li, Z. Li, Q. Luo, H. Zhu, Z. Gu, H. Zhang, Q. Gong, K. Luo, Appl. Mater. Today 20, 100686 (2020)
W. He, S. Li, L. Wang, L. Zhu, Y. Zhang, Y. Luo, K. Huang, W. Xu, Sens. Actuators B: Chem. 290, 503 (2019)
Z.-M. Chang, H. Zhou, C. Yang, R. Zhang, Q. You, R. Yan, L. Li, M. Ge, Y. Tang, W.-F. Dong, J. Mater. Chem. B 8, 5019 (2020)
S. Wu, L. Gu, J. Qin, L. Zhang, F. Sun, Z. Liu, Y. Wang, D. Shi, A.C.S. Appl, Mater. Interfaces 12, 4193 (2020)
P. Ding, Z. Wang, Z. Wu, M. Hu, W. Zhu, N. Sun, R. Pei, A.C.S. Appl, Mater. Interfaces 13, 3694 (2021)
S. Aggarwal, Nat. Rev. Drug Discov. 9, 427 (2010)
D. Woods, J.J. Turchi, Cancer Biol. Ther. 14, 379 (2013)
E. Smart, F. Lopes, S. Rice, B. Nagy, R.A. Anderson, R.T. Mitchell, N. Spears, Sci. Rep. 8, 1 (2018)
A. Pearce, M. Haas, R. Viney, S.-A. Pearson, P. Haywood, C. Brown, R. Ward, PLoS ONE 12, e0184360 (2017)
D. Mossmann, S. Park, M.N. Hall, Nat. Rev. Cancer 18, 744 (2018)
A.R. Moore, S.C. Rosenberg, F. McCormick, S. Malek, Nat. Rev. Drug Discov. 19, 533 (2020)
Y.T. Lee, Y.J. Tan, C.E. Oon, Eur. J. Pharmacol. 834, 188 (2018)
F.R. Greten, S.I. Grivennikov, Immunity 51, 27 (2019)
K.-Q. Yang, Y. Liu, Q.-H. Huang, N. Mo, Q.-Y. Zhang, Q.-G. Meng, J.-W. Cheng, BMC Cancer 17, 1 (2017)
P. Nandi, G.V. Girish, M. Majumder, X. **n, E. Tutunea-Fatan, P.K. Lala, BMC Cancer 17, 1 (2017)
N. Maishi, K. Hida, Cancer Sci. 108, 1921 (2017)
E. Sahai, I. Astsaturov, E. Cukierman, D.G. DeNardo, M. Egeblad, R.M. Evans, D. Fearon, F.R. Greten, S.R. Hingorani, T. Hunter, Nat. Rev. Cancer 20, 174 (2020)
G. Hoxhaj, B.D. Manning, Nat. Rev. Cancer 20, 74 (2020)
J. Yuan, W.H. Ng, Z. Tian, J. Yap, M. Baccarini, Z. Chen, J. Hu, Sci. Signal. 11, eaar6795 (2018)
R. Barbosa, L.A. Acevedo, R. Marmorstein, Mol. Cancer Res. 19, 361 (2021)
M.B. Ryan, R.B. Corcoran, Nat. Rev. Clin. Oncol. 15, 709 (2018)
P Timmins, Ther. Deliv. (2021).
G. Pines, W.J. Köstler, Y. Yarden, FEBS Lett. 584, 2699 (2010)
X. Gao, X. **a, F. Li, M. Zhang, H. Zhou, X. Wu, J. Zhong, Z. Zhao, K. Zhao, D. Liu, F. **ao, Q. Xu, T. Jiang, B. Li, S.-Y. Cheng, N. Zhang, Nat. Cell Biol. 23, 278 (2021)
P. Workman, G.F. Draetta, J.H. Schellens, R. Bernards, Cell 168, 579 (2017)
L.M. McNamee, M.J. Walsh, F.D. Ledley, PLoS ONE 12, e0177371 (2017)
C.C. Bell, O. Gilan, Br. J. Cancer 122, 465 (2020)
Z.C. Nwosu, W. Piorońska, N. Battello, A.D. Zimmer, B. Dewidar, M. Han, S. Pereira, B. Blagojevic, D. Castven, V. Charlestin, EBioMedicine 54, 102699 (2020)
S. K. Gupta, P. Singh, V. Ali, M. Verma, Oncol. Rev. 14, 448 (2020)
R.A. Ward, S. Fawell, N. Floc’h, V. Flemington, D. McKerrecher, P.D. Smith, Chem. Rev. 121(6), 3297–3351 (2020)
M. Carceles-Cordon, W.K. Kelly, L. Gomella, K.E. Knudsen, V. Rodriguez-Bravo, J. Domingo-Domenech, Nat. Rev. Urol. 17, 292 (2020)
V. Kushwah, S.S. Katiyar, C.P. Dora, A.K. Agrawal, D.A. Lamprou, R.C. Gupta, S. Jain, Acta Biomater. 73, 424 (2018)
A.C. Krauss, X. Gao, L. Li, M.L. Manning, P. Patel, W. Fu, K.G. Janoria, G. Gieser, D.A. Bateman, D. Przepiorka, Clin. Cancer Res. 25, 2685 (2019)
W.-S. Lim, P.G. Tardi, N. Dos Santos, X. **e, M. Fan, B.D. Liboiron, X. Huang, T.O. Harasym, D. Bermudes, L.D. Mayer, Leuk. Res. 34, 1214 (2010)
M.S. Goldberg, Nat. Rev. Cancer 19, 587 (2019)
M. Craig, A.L. Jenner, B. Namgung, L.P. Lee, A. Goldman, Chem. Rev. 121(6), 3352–3389 (2020)
F.M. Frame, A.R. Noble, S. Klein, H.F. Walker, R. Suman, R. Kasprowicz, V.M. Mann, M.S. Simms, N.J. Maitland, J Cancer Metastasis Treat 3, 302 (2017)
M. Piffoux, A.K. Silva, C. Wilhelm, F. Gazeau, D. Tareste, ACS Nano 12, 6830 (2018)
Z. Cai, Y. Zhang, Z. He, L.-P. Jiang, J.-J. Zhu, ACS Appl. Bio Mater. 3, 5322 (2020)
Y. Zhen, K.K. Ewert, W.S. Fisher, V.M. Steffes, Y. Li, C.R. Safinya, Sci. Rep. 11, 7311 (2021)
G. Ciarimboli, Anticancer Res. 34, 547 (2014)
J. E. Larsen, J. R. Henriksen, M. Bæksted, T. L. Andresen, G. K. Jacobsen, K. Jørgensen, Clin. Cancer. Res. 12, 19 (2006)
B.S. Cummings, Biochem. Pharmacol. 74, 949 (2007)
E.H. Jakobsen, D. Nielsen, H. Danoe, S. Linnet, J. Hansen, U.N. Lassen, E. Balslev, V. Glavicic, J. Bogovic, S. Knudsen, B. Ejlertsen, A.S. Knoop, U.H. Buhl, M.W. Madsen, I.K. Buhl, A. Hansen, T. Jensen, A. Rasmussen, P.B. Jensen, S.T. Langkjer, J. Clin. Oncol. 36, e13077 (2018)
B. Hakyemez, C. Erdogan, I. Ercan, N. Ergin, S. Uysal, S. Atahan, Clin. Radiol. 60, 493 (2005)
D. Jayamanne, H. Wheeler, R. Cook, C. Teo, D. Brazier, G. Schembri, M. Kastelan, L. Guo, M.F. Back, ANZ J. Surg. 88, 196 (2018)
L Nayak, K Panageas, L Deangelis, L Abrey, A Lassman, Neuro Oncol. 12 (2010).
C.L. Grek, Z. Sheng, C.C. Naus, W.C. Sin, R.G. Gourdie, G.G. Ghatnekar, Curr. Opin. Pharmacol. 41, 79 (2018)
A. Mehta, A. Sonabend, J. Bruce, Neurotherapeutics 14, 358 (2017)
Y. Yu, C. DesJardins, P. Saxton, G. Lai, E. Schuck, Y.N. Wong, Int. J. Pharm. 443, 9 (2013)
Y. Dou, K. Hynynen, C. Allen, J. Control. Release 249, 63 (2017)
F. Wang, M. Porter, A. Konstantopoulos, P. Zhang, H. Cui, J. Control. Release 267, 100 (2017)
L. Kiss, F.R. Walter, A. Bocsik, S. Veszelka, B. Ózsvári, L.G. Puskás, P. Szabó-Révész, M.A. Deli, J. Pharm. Sci. 102, 1173 (2013)
J. Riedel, M.N. Calienni, E. Bernabeu, V. Calabro, J.M. Lázaro-Martinez, M.J. Prieto, L. Gonzalez, C.S. Martinez, S d V Alonso, J Montanari, P Evelson, D A Chiappetta, M A Moretton. J. Drug Deliv. Sci. Technol. 62, 102343 (2021)
A. Varela-Moreira, Y. Shi, M.H. Fens, T. Lammers, W.E. Hennink, R.M. Schiffelers, Mater Chem Front 1, 1485 (2017)
J. Liu, F. Zeng, C. Allen, J. Control. Release 103, 481 (2005)
Y. Lu, E. Zhang, J. Yang, Z. Cao, Nano Res. 11, 4985 (2018)
I. Ekladious, Y.L. Colson, M.W. Grinstaff, Nat. Rev. Drug Discov. 18, 273 (2019)
M.L. Girase, P.G. Patil, P.P. Ige, Int. J. Polym. Mater. Polym. Biomater. 69, 990 (2020)
M.B. Ashford, R.M. England, N. Akhtar, Adv. Ther. 4(5), 2000285 (2021)
S. Xu, L. Wang, Z. Liu, Angew. Chem. Int. Ed. 60, 3858 (2021)
S.M. Stavis, J.A. Fagan, M. Stopa, J.A. Liddle, ACS Appl. Nano Mater. 1, 4358 (2018)
S. Gaur, L. Chen, T. Yen, Y. Wang, B. Zhou, M. Davis, Y. Yen, Nanomed. Nanotechnol. Biol. Med. 8, 721 (2012)
T. Numbenjapon, J. Wang, D. Colcher, T. Schluep, M.E. Davis, J. Duringer, L. Kretzner, Y. Yen, S.J. Forman, A. Raubitschek, Clin. Cancer Res. 15, 4365 (2009)
T. Schluep, J. Hwang, I.J. Hildebrandt, J. Czernin, C.H.J. Choi, C.A. Alabi, B.C. Mack, M.E. Davis, Proc. Natl. Acad. Sci. 106, 11394 (2009)
J.C. Reubi, L. Mazzucchelli, I. Hennig, J.A. Laissue, Gastroenterology 110, 1719 (1996)
A. Parodi, J. Miao, S.M. Soond, M. Rudzińska, A.A. Zamyatnin, Biomolecules 9, 218 (2019)
L. Mocan, C. Matea, F.A. Tabaran, O. Mosteanu, T. Pop, T. Mocan, C. Iancu, Int. J. Nanomedicine 10, 5435 (2015)
H. Yuan, H. Guo, X. Luan, M. He, F. Li, J. Burnett, N. Truchan, D. Sun, Mol. Pharm. 17, 2275 (2020)
S. Sau, H.O. Alsaab, S.K. Kashaw, K. Tatiparti, A.K. Iyer, Drug Discovery Today 22, 1547 (2017)
C.H. Chau, P.S. Steeg, W.D. Figg, The Lancet 394, 793 (2019)
J.Z. Drago, S. Modi, S. Chandarlapaty, Nat. Rev. Clin. Oncol. 18, 327 (2021)
R. Ubink, E.H. Dirksen, M. Rouwette, E.S. Bos, I. Janssen, D.F. Egging, E.M. Loosveld, T.A. van Achterberg, K. Berentsen, M.M. van der Lee, Mol. Cancer Ther. 17, 2389 (2018)
G. Lenz, R.E. Davis, V.N. Ngo, L. Lam, T.C. George, G.W. Wright, S.S. Dave, H. Zhao, W. Xu, A. Rosenwald, Science 319, 1676 (2008)
A. Lee, Drugs, 81, 1229 (2021)
S. Wahby, L. Fashoyin-Aje, C.L. Osgood, J. Cheng, M.H. Fiero, L. Zhang, S. Tang, S.S. Hamed, P. Song, R. Charlab, Clin. Cancer Res. 27, 1850 (2021)
A. Bardia, Clin Adv Hematol Oncol H&O 18, 715 (2020)
M.-G. Kim, Y. Shon, J. Kim, Y.-K. Oh, J. Natl. Cancer Inst. 109, djw186 (2017)
W. Wang, Q. Sun, Sig Transduct Target Ther 5, 65 (2020)
P.L. Stern, Cancer Immunology (Springer, 2021), p. 413
S.A. Kerk, K.A. Finkel, A.T. Pearson, K.A. Warner, Z. Zhang, F. Nör, V.P. Wagner, P.A. Vargas, M.S. Wicha, E.M. Hurt, Clin. Cancer Res. 23, 2516 (2017)
R.G. Coumans, G.J. Ariaans, H.J. Spijker, P. Renart Verkerk, P.H. Beusker, B.P. Kokke, J. Schouten, M. Blomenröhr, M.M. van der Lee, P.G. Groothuis, Bioconjug. Chem. 31, 2136 (2020)
Z. Zhou, X. Liu, D. Zhu, Y. Wang, Z. Zhang, X. Zhou, N. Qiu, X. Chen, Y. Shen, Adv. Drug Del. Rev. 115, 115 (2017)
M.L. Lugin, R.T. Lee, Y.J. Kwon, ACS Nano 14, 14262 (2020)
K.-L. Muir, A. Kallam, S.A. Koepsell, K. Gundabolu, N. Engl, J. Med. 384, 1964 (2021)
D W Eyre, S F Lumley, J Wei, S Cox, T James, A Justice, G Jesuthasan, D O’Donnell, A Howarth, S B Hatch, Clin Microbiol Infect (2021).
A.D. Judge, M. Robbins, I. Tavakoli, J. Levi, L. Hu, A. Fronda, E. Ambegia, K. McClintock, I. MacLachlan, J. Clin. Investig. 119, 661 (2009)
J. Huang, D. **ao, G. Li, J. Ma, P. Chen, W. Yuan, F. Hou, J. Ge, M. Zhong, Y. Tang, Oncogene 33, 2737 (2014)
I. Fabregat, D. Caballero-Díaz, Front. Oncol. 8, 357 (2018)
M. Molyneaux, J. Xu, D. M. Evans, P. Lu, Am. J. Clin. Oncol. 37, 15 (2019)
T. Rimkus, S. Sirkisoon, A. Harrison, H.-W. Lo, Discov. Med. 23, 325 (2017)
C. Lu, D.J. Stewart, J.J. Lee, L. Ji, R. Ramesh, G. Jayachandran, M.I. Nunez, I.I. Wistuba, J.J. Erasmus, M.E. Hicks, PLoS ONE 7, e34833 (2012)
B. Dai, S. Yan, H. Lara-Guerra, H. Kawashima, R. Sakai, G. Jayachandran, M. Majidi, R. Mehran, J. Wang, B.N. Bekele, PLoS ONE 10, e0123967 (2015)
S.P. Chawla, H. Bruckner, M.A. Morse, N. Assudani, F.L. Hall, E.M. Gordon, Mol. Ther. Oncolytics 12, 56 (2019)
A. Al-Shihabi, S.P. Chawla, F.L. Hall, E.M. Gordon, Mol. Ther. Oncolytics 11, 122 (2018)
E.M. Gordon, J.R. Ravicz, S. Liu, S.P. Chawla, F.L. Hall, Mol. Clin. Oncol. 9, 115 (2018)
J Ravicz, C Szeto, S Reddy, S Chawla, M Morse, E Gordon, Preprints (2021).
B. R. Champion, M. Besneux, M. Patsalidou, A. Silva, M. Zonca, N. Marino, G. Di Genova, S. Illingworth, S. Fedele, L. Slater, Cancer Res. 79, 13 (2019)
L. Menotti, E. Avitabile, Int. J. Mol. Sci. 21, 8310 (2020)
J.D. Bernstock, S.E. Hoffman, J.A. Chen, S. Gupta, A.D. Kappel, T.R. Smith, E.A. Chiocca, Viruses 13, 1158 (2021)
I. Watanabe, H. Kasuya, N. Nomura, T. Shikano, T. Shirota, N. Kanazumi, S. Takeda, S. Nomoto, H. Sugimoto, A. Nakao, Cancer Chemother. Pharmacol. 61, 875 (2008)
R. Gao, X. Yan, C. Zheng, C.M. Goldsmith, S. Afione, B. Hai, J. Xu, J. Zhou, C. Zhang, J.A. Chiorini, Gene Ther. 18, 38 (2011)
G. Van Niel, G. d’Angelo, G. Raposo, Nat. Rev. Mol. Cell Biol. 19, 213 (2018)
T.H. Ung, H.J. Madsen, J.E. Hellwinkel, A.M. Lencioni, M.W. Graner, Cancer Sci. 105, 1384 (2014)
M.P. Bebelman, P. Bun, S. Huveneers, G. van Niel, D.M. Pegtel, F.J. Verweij, Nat. Protoc. 15, 102 (2020)
G. Kibria, E.K. Ramos, Y. Wan, D.R. Gius, H. Liu, Mol. Pharm. 15, 3625 (2018)
S. Kamerkar, V.S. LeBleu, H. Sugimoto, S. Yang, C.F. Ruivo, S.A. Melo, J.J. Lee, R. Kalluri, Nature 546, 498 (2017)
N. Gildener-Leapman, R.L. Ferris, J.E. Bauman, Oral Oncol. 49, 1089 (2013)
J. Jou, K.J. Harrington, M.-B. Zocca, E. Ehrnrooth, E.E. Cohen, Clin. Cancer Res. 27, 689 (2021)
W.-L. Liu, M.-Z. Zou, T. Liu, J.-Y. Zeng, X. Li, W.-Y. Yu, C.-X. Li, J.-J. Ye, W. Song, J. Feng, Nat. Commun. 10, 1 (2019)
H.J. Stauss, E.C. Morris, H. Abken, Curr. Opin. Pharmacol. 24, 113 (2015)
R. Thomas, G. Al-Khadairi, J. Roelands, W. Hendrickx, S. Dermime, D. Bedognetti, J. Decock, Front. Immunol. 9, 947 (2018)
P.Y. Lam, M.D. Nissen, S.R. Mattarollo, Front. Immunol. 8, 1355 (2017)
K. Oleinika, E.C. Rosser, D.E. Matei, K. Nistala, A. Bosma, I. Drozdov, C. Mauri, Nat. Commun. 9, 684 (2018)
S.W. Lee, H.J. Park, L. Van Kaer, S. Hong, S. Hong, Sci. Rep. 8, 1 (2018)
Y.J. Lee, H. Wang, G.J. Starrett, V. Phuong, S.C. Jameson, K.A. Hogquist, Immunity 43, 566 (2015)
J. Yang, S. Arya, P. Lung, Q. Lin, J. Huang, Q. Li, Nanoscale 11, 21782 (2019)
E. Maraskovsky, S. Sjölander, D.P. Drane, M. Schnurr, T.T. Le, L. Mateo, T. Luft, K.-A. Masterman, T.-Y. Tai, Q. Chen, Clin. Cancer Res. 10, 2879 (2004)
O. Gasser, K.J. Sharples, C. Barrow, G.M. Williams, E. Bauer, C.E. Wood, B. Mester, M. Dzhelali, G. Caygill, J. Jones, Cancer Immunol. Immunother. 67, 285 (2018)
Y. Dölen, U. Gileadi, J.-L. Chen, M. Valente, J.H. Creemers, E.A. Van Dinther, N.K. van Riessen, E. Jäger, M. Hruby, V. Cerundolo, Front. Immunol. 12, 372 (2021)
D.V. Parums, Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 27, e933088 (2021)
Z. Jahanafrooz, B. Baradaran, J. Mosafer, M. Hashemzaei, T. Rezaei, A. Mokhtarzadeh, M.R. Hamblin, Drug Discov. Today 25, 552 (2020)
A. Mullard, Nat. Rev. Drug Discov. 15, 663 (2016)
J.L. Tanyi, S. Bobisse, E. Ophir, S. Tuyaerts, A. Roberti, R. Genolet, P. Baumgartner, B.J. Stevenson, C. Iseli, D. Dangaj, Sci. Transl. Med. 10, eaao5931 (2018)
E. Dolgin, Nature 574, S10 (2019)
H A Burris, M R Patel, D C Cho, J M Clarke, M Gutierrez, T Z Zaks, J Frederick, K Hopson, K Mody, A Binanti-Berube, Am. J. Clin. Oncol., 2019.
L.M. Kranz, M. Diken, H. Haas, S. Kreiter, C. Loquai, K.C. Reuter, M. Meng, D. Fritz, F. Vascotto, H. Hefesha, Nature 534, 396 (2016)
U. Sahin, P. Oehm, E. Derhovanessian, R.A. Jabulowsky, M. Vormehr, M. Gold, D. Maurus, D. Schwarck-Kokarakis, A.N. Kuhn, T. Omokoko, Nature 585, 107 (2020)
Y. Mi, C.T. Hagan IV., B.G. Vincent, A.Z. Wang, Adv. 6, 1801847 (2019)
M.H. Tuthill, A. Montinaro, J. Zinngrebe, K. Prieske, P. Draber, S. Prieske, T. Newsom-Davis, S. von Karstedt, J. Graves, H. Walczak, Oncogene 34, 2138 (2015)
S.M. Lim, T.H. Kim, H.H. Jiang, C.W. Park, S. Lee, X. Chen, K.C. Lee, Biomaterials 32, 3538 (2011)
P. Zhao, X. Tang, Y. Huang, View 2(4), 20200127 (2021)
C.X. Ma, S. Cai, S. Li, C.E. Ryan, Z. Guo, W.T. Schaiff, L. Lin, J. Hoog, R.J. Goiffon, A. Prat, J. Clin. Investig. 122, 1541 (2012)
T.T. Byrd, K. Fousek, A. Pignata, C. Szot, H. Samaha, S. Seaman, L. Dobrolecki, V.S. Salsman, H.Z. Oo, K. Bielamowicz, Cancer Res. 78, 489 (2018)
J. Xu, Y. Liu, Y. Li, H. Wang, S. Stewart, K. Van der Jeught, P. Agarwal, Y. Zhang, S. Liu, G. Zhao, J. Wan, X. Lu, X. He, Nat. Nanotechnol. 14, 388 (2019)
C.-T. Jiang, K.-G. Chen, A. Liu, H. Huang, Y.-N. Fan, D.-K. Zhao, Q.-N. Ye, H.-B. Zhang, C.-F. Xu, S. Shen, M.-H. **ong, J.-Z. Du, X.-Z. Yang, J. Wang, Nat. Commun. 12, 1359 (2021)
R.C. YashRoy, Nanostructures for Antimicrobial Therapy (Elsevier, 2017), p. 341
M.J. Gerritzen, D.E. Martens, R.H. Wijffels, L. van der Pol, M. Stork, Biotechnol. Adv. 35, 565 (2017)
K. Cheng, R. Zhao, Y. Li, Y. Qi, Y. Wang, Y. Zhang, H. Qin, Y. Qin, L. Chen, C. Li, J. Liang, Y. Li, J. Xu, X. Han, G.J. Anderson, J. Shi, L. Ren, X. Zhao, G. Nie, Nat. Commun. 12, 2041 (2021)
M. Huo, H. Wang, Y. Zhang, H. Cai, P. Zhang, L. Li, J. Zhou, T. Yin, J. Control. Release 321, 198 (2020)
Z.Y. Xu-Monette, M. Zhang, J. Li, K.H. Young, Front. Immunol. 8, 1597 (2017)
J. Liu, Z. Zhao, N. Qiu, Q. Zhou, G. Wang, H. Jiang, Y. Piao, Z. Zhou, J. Tang, Y. Shen, Nat. Commun. 12, 1 (2021)
B.-B. Zhang, X.-J. Chen, X.-D. Fan, J.-J. Zhu, Y.-H. Wei, H.-S. Zheng, H.-Y. Zheng, B.-H. Wang, J.-G. Piao, F.-Z. Li, Acta Pharmacol. Sin. 42, 832 (2021)
X. Wei, J. Yang, S.J. Adair, H. Ozturk, C. Kuscu, K.Y. Lee, W.J. Kane, P.E. O’Hara, D. Liu, Y.M. Demirlenk, Proc. Natl. Acad. Sci. 117, 28068 (2020)
D. Rosenblum, A. Gutkin, R. Kedmi, S. Ramishetti, N. Veiga, A.M. Jacobi, M.S. Schubert, D. Friedmann-Morvinski, Z.R. Cohen, M.A. Behlke, Sci. Adv. 6, eabc9450 (2020)
S. Deng, X. Li, S. Liu, J. Chen, M. Li, S.Y. Chew, K.W. Leong, D. Cheng, Sci. Adv. 6, eabb4005 (2020)
A. Mullard, Nat. Rev. Drug Discov. 13, 877 (2014)
X. Liang, P. Wu, Q. Yang, Y. **e, C. He, L. Yin, Z. Yin, G. Yue, Y. Zou, L. Li, Eur. J. Med. Chem. 220, 113473 (2021)
T.M. Govers, D. Hessels, V. Vlaeminck-Guillem, B.J. Schmitz-Dräger, C.G. Stief, C. Martinez-Ballesteros, M. Ferro, A. Borque-Fernando, J. Rubio-Briones, J.M. Sedelaar, Prostate Cancer Prostatic Dis. 22, 101 (2019)
D. Spano, C. Heck, P. De Antonellis, G. Christofori, M. Zollo, Seminars in Cancer Biology (Elsevier, 2012), p. 234
Z. Kakushadze, R. Raghubanshi, W. Yu, Data 2, 30 (2017)
M.A. Rossing, K.G. Wicklund, K.L. Cushing-Haugen, N.S. Weiss, J. Natl. Cancer Inst. 102, 222 (2010)
W. Park, J. Chen, J.F. Chou, A.M. Varghese, H.Y. Kenneth, W. Wong, M. Capanu, V. Balachandran, C.A. McIntyre, I. El Dika, Clin. Cancer Res. 26, 3239 (2020)
S. Srivastava, E.J. Koay, A.D. Borowsky, A.M. De Marzo, S. Ghosh, P.D. Wagner, B.S. Kramer, Nat. Rev. Cancer 19, 349 (2019)
F.M. Walter, J.D. Emery, S. Mendonca, N. Hall, H.C. Morris, K. Mills, C. Dobson, C. Bankhead, M. Johnson, G.A. Abel, Br. J. Cancer 115, 533 (2016)
M.F. Vine, B. Calingaert, A. Berchuck, J.M. Schildkraut, Gynecol. Oncol. 90, 75 (2003)
A. Umar, S. Atabo, M.O.J. Anat, Physiol. 6, 175 (2019)
L.G. Koss, JAMA 261, 737 (1989)
D.H. Greegor, Cancer 28, 131 (1971)
B. Holmström, M. Johansson, A. Bergh, U.-H. Stenman, G. Hallmans, P. Stattin, BMJ 339, b3537 (2009)
T.B. Bevers, B.O. Anderson, E. Bonaccio, S. Buys, M.B. Daly, P.J. Dempsey, W.B. Farrar, I. Fleming, J.E. Garber, R.E. Harris, J. Natl. Compr. Canc. Netw. 7, 1060 (2009)
D. Provenzale, K. Jasperson, D.J. Ahnen, H. Aslanian, T. Bray, J.A. Cannon, D.S. David, D.S. Early, D. Erwin, J.M. Ford, J. Natl. Compr. Canc. Netw. 13, 959 (2015)
D.E. Wood, G.A. Eapen, D.S. Ettinger, L. Hou, D. Jackman, E. Kazerooni, D. Klippenstein, R.P. Lackner, L. Leard, A.N. Leung, J. Natl. Compr. Canc. Netw. 10, 240 (2012)
Z. Zhou, M. Qutaish, Z. Han, R.M. Schur, Y. Liu, D.L. Wilson, Z.-R. Lu, Nat. Commun. 6, 1 (2015)
Q.T. Nguyen, E.S. Olson, T.A. Aguilera, T. Jiang, M. Scadeng, L.G. Ellies, R.Y. Tsien, Proc. Natl. Acad. Sci. 107, 4317 (2010)
S. He, J. Li, M. Chen, L. Deng, Y. Yang, Z. Zeng, W. **ong, X. Wu, Int. J. Nanomedicine 15, 8451 (2020)
M.M. Calabretta, M. Zangheri, A. Lopreside, E. Marchegiani, L. Montali, P. Simoni, A. Roda, Analyst 145, 2841 (2020)
E. Stern, A. Vacic, N.K. Rajan, J.M. Criscione, J. Park, B.R. Ilic, D.J. Mooney, M.A. Reed, T.M. Fahmy, Nat. Nanotechnol. 5, 138 (2010)
A. Dart, Nat. Rev. Cancer 20, 299 (2020)
X.-X. Jie, X.-Y. Zhang, C.-J. Xu, Oncotarget 8, 81558 (2017)
J. Chen, C. Ye, J. Dong, S. Cao, Y. Hu, B. Situ, X. **, S. Qin, J. Xu, Z. Cai, J. Transl. Med. 18, 1 (2020)
T. Pereira-Veiga, M. González-Conde, L. León-Mateos, R. Piñeiro-Cid, C. Abuín, L. Muinelo-Romay, M. Martínez-Fernández, J.B. Iglesias, J.G. González, U. Anido, Clin. Exp. Metastasis 38, 239 (2021)
X. Hu, X. Zang, Y. Lv, Oncol. Lett. 21, 1 (2021)
J. C. Ahn, P. C. Teng, P. J. Chen, E. Posadas, H. R. Tseng, S. C. Lu, J. D. Yang, Hepatology, 73, 422 (2020)
L.M. Russell, C.H. Liu, P. Grodzinski, Biomaterials 242, 119926 (2020)
X. Wang, S. Cheng, X. Wang, L. Wei, Q. Kong, M. Ye, X. Luo, J. Xu, C. Zhang, Y. **an, ACS Sens. 6, 1925 (2021)
L. Zhu, X. Feng, S. Yang, J. Wang, Y. Pan, J. Ding, C. Li, X. Yin, Y. Yu, Biosens. Bioelectron. 172, 112780 (2021)
J. Dong, J.F. Chen, M. Smalley, M. Zhao, Z. Ke, Y. Zhu, H.R. Tseng, Adv. Mater. 32, 1903663 (2020)
Z. Li, G. Wang, Y. Shen, N. Guo, N. Ma, Adv. Funct. Mater. 28, 1707152 (2018)
C. Patriarca, R.M. Macchi, A.K. Marschner, H. Mellstedt, Cancer Treat. Rev. 38, 68 (2012)
L.E. Lowes, D. Goodale, Y. **a, C. Postenka, M.M. Piaseczny, F. Paczkowski, A.L. Allan, Oncotarget 7, 76125 (2016)
J. Kitz, D. Goodale, C. Postenka, L.E. Lowes, A.L. Allan, Clin. Exp. Metastasis 38, 97 (2021)
K. Campbell, F. Rossi, J. Adams, I. Pitsidianaki, F.M. Barriga, L. Garcia-Gerique, E. Batlle, J. Casanova, A. Casali, Nat. Commun. 10, 1 (2019)
D. Ribatti, R. Tamma, T. Annese, Transl. Oncol. 13, 100773 (2020)
A. Philippron, L. Depypere, S. Oeyen, B. De Laere, C. Vandeputte, P. Nafteux, K. De Preter, P. Pattyn, PLoS ONE 16, e0251052 (2021)
C. A. LaBelle, A. Massaro, B. Cortés-Llanos, C. E. Sims, N. L. Allbritton, Trends Biotechnol. 39, 613 (2020)
S.V. Puram, I. Tirosh, A.S. Parikh, A.P. Patel, K. Yizhak, S. Gillespie, C. Rodman, C.L. Luo, E.A. Mroz, K.S. Emerick, Cell 171, 1611 (2017)
R.M. Hoffman, F.D. Gilliland, M. Adams-Cameron, W.C. Hunt, C.R. Key, B.M.C. Fam, Pract. 3, 1 (2002)
H. Kim, S. Park, I.G. Jeong, S.H. Song, Y. Jeong, C.-S. Kim, K.H. Lee, ACS Nano 15, 4054 (2021)
V.P. Jayaseelan, Cancer Gene Ther. 27, 395 (2020)
V.S. LeBleu, R. Kalluri, Trends Cancer 6(9), 769–774 (2020)
B. Zhou, K. Xu, X. Zheng, T. Chen, J. Wang, Y. Song, Y. Shao, S. Zheng, Signal Transduct. Target. Ther. 5, 1 (2020)
A. Perez-Cornago, P.N. Appleby, T. Pischon, K.K. Tsilidis, A. Tjønneland, A. Olsen, K. Overvad, R. Kaaks, T. Kühn, H. Boeing, BMC Med. 15, 1 (2017)
A. Amann, B. de LacyCostello, W. Miekisch, J. Schubert, B. Buszewski, J. Pleil, N. Ratcliffe, T. Risby, J. Breath Res. 8, 034001 (2014)
M.A.G. Wallace, J.D. Pleil, Anal. Chim. Acta 1024, 18 (2018)
R. Thriumani, A. Zakaria, Y.Z.H.-Y. Hashim, A.I. Jeffree, K.M. Helmy, L.M. Kamarudin, M.I. Omar, A.Y.M. Shakaff, A.H. Adom, K.C. Persaud, BMC Cancer 18, 1 (2018)
C. Baldini, L. Billeci, F. Sansone, R. Conte, C. Domenici, A. Tonacci, Biosensors 10, 84 (2020)
M.P. Fernandes, S. Venkatesh, B. Sudarshan, Open Biomed. Eng. J. 9, 228 (2015)
M. Senba, N. Mori, Oncol. Rev. 6, 2 (2012)
B.C. Sheu, W.C. Chang, H.H. Lin, S.N. Chow, S.C. Huang, J. Obstet. Gynaecol. Res. 33, 103 (2007)
R. Gupta, P.L. Rady, H.Q. Doan, S.K. Tyring, J. Clin. Virol. 126, 104348 (2020)
T.J. Baek, P.Y. Park, K.N. Han, H.T. Kwon, G.H. Seong, Anal. Bioanal. Chem. 390, 1373 (2008)
R. Bassan, O. Spinelli, E. Oldani, T. Intermesoli, M. Tosi, B. Peruta, G. Rossi, E. Borlenghi, E.M. Pogliani, E. Terruzzi, Blood 113, 4153 (2009)
R. Vinhas, R. Mendes, A.R. Fernandes, P.V. Baptista, Front. Bioeng. Biotechnol. 5, 79 (2017)
J.E. Jaetao, K.S. Butler, N.L. Adolphi, D.M. Lovato, H.C. Bryant, I. Rabinowitz, S.S. Winter, T.E. Tessier, H.J. Hathaway, C. Bergemann, Cancer Res. 69, 8310 (2009)
Y. Nakamura, H. Yasuoka, M. Tsujimoto, K. Kurozumi, M. Nakahara, K. Nakao, K. Kakudo, J. Clin. Pathol. 59, 77 (2006)
O. Suzuki, Y. Sekishita, T. Shiono, K. Ono, M. Fujimori, S. Kondo, J. Am. Coll. Surg. 202, 732 (2006)
M.G. Harisinghani, J. Barentsz, P.F. Hahn, W.M. Deserno, S. Tabatabaei, C.H. van de Kaa, J. de la Rosette, R. Weissleder, N. Engl, J. Med. 348, 2491 (2003)
P. Bader, F.C. Burkhard, R. Markwalder, U.E. Studer, J. Urol. 168, 514 (2002)
H.J.M. Meijer, A.S. Fortuin, E.N.J.T. van Lin, O.A. Debats, J. Alfred Witjes, J.H.A.M. Kaanders, J.O. Barentsz, Radiother. Oncol. 106, 59 (2013)
R.A. Heesakkers, G.J. Jager, A.M. Hovels, B. de Hoop, H.C. van den Bosch, F. Raat, J.A. Witjes, P.F. Mulders, C.H. van der Kaa, J.O. Barentsz, Radiology 251, 408 (2009)
A.M. Hövels, R.A.M. Heesakkers, E.M. Adang, G.J. Jager, S. Strum, Y.L. Hoogeveen, J.L. Severens, J.O. Barentsz, Clin. Radiol. 63, 387 (2008)
T.F. Wood, D.T. Nora, D.L. Morton, R.R. Turner, D. Rangel, W. Hutchinson, A.J. Bilchik, J. Gastrointest. Surg. 6, 322 (2002)
M.G. Bredell, Head Neck. Oncol. 2, 1 (2010)
D. Murawa, C. Hirche, S. Dresel, M. Hünerbein, Br. J. Surg. 96, 1289 (2009)
T.K. Hill, A. Abdulahad, S.S. Kelkar, F.C. Marini, T.E. Long, J.M. Provenzale, A.M. Mohs, Bioconjug. Chem. 26, 294 (2015)
M.S. Bradbury, E. Phillips, P.H. Montero, S.M. Cheal, H. Stambuk, J.C. Durack, C.T. Sofocleous, R.J. Meester, U. Wiesner, S. Patel, Integr. Biol. 5, 74 (2013)
M. G. Schilham, P. Zamecnik, B. M. Privé, B. Israël, M. Rijpkema, T. Scheenen, J. O. Barentsz, J. Nagarajah, M. Gotthardt, J. Nucl. Med. 62, 10 (2021)
Z. Chen, M. Chen, K. Zhou, J. Rao, Angew. Chem. Int. Ed. 59, 7864 (2020)
T. Martone, A. Gillio-Tos, L. De Marco, V. Fiano, M. Maule, A. Cavalot, M. Garzaro, F. Merletti, G. Cortesina, Clin. Cancer Res. 13, 5089 (2007)
M. Alicandri-Ciufelli, M. Bonali, A. Piccinini, L. Marra, A. Ghidini, E.M. Cunsolo, A. Maiorana, L. Presutti, P.F. Conte, Eur. Arch. Otorhinolaryngol. 270, 2603 (2013)
A. Becker, C. Hessenius, K. Licha, B. Ebert, U. Sukowski, W. Semmler, B. Wiedenmann, C. Grötzinger, Nat. Biotechnol. 19, 327 (2001)
C.N. Loynachan, A.P. Soleimany, J.S. Dudani, Y. Lin, A. Najer, A. Bekdemir, Q. Chen, S.N. Bhatia, M.M. Stevens, Nat. Nanotechnol. 14, 883 (2019)
H. R. Salinas, D. L. Miyasato, O. E. Eremina, R. Perez, K. L. Gonzalez, A. T. Czaja, S. Burkitt, A. Aron, A. Fernando, L. S. Ojeda, Biomater. Sci. 9, 482 (2020)
J. Larkin, R.Y. Henley, V. Jadhav, J. Korlach, M. Wanunu, Nat. Nanotechnol. 12, 1169 (2017)
T. Laver, J. Harrison, P. O’neill, K. Moore, A. Farbos, K. Paszkiewicz, D.J. Studholme, Biomol. Detect. Quantif. 3, 1 (2015)
Y. Van Der Pol, F. Mouliere, Cancer Cell 36, 350 (2019)
P. Liu, R. Kawano, Small Methods 4, 2000101 (2020)
J. Zhou, Z. Wu, J. Hu, D. Yang, X. Chen, Q. Wang, J. Liu, M. Dou, W. Peng, Y. Wu, Sci. Adv. 6, eabc1204 (2020)
S. Pan, Y. Zhang, A. Natalia, C.Z.J. Lim, N.R.Y. Ho, B. Chowbay, T.P. Loh, J.K.C. Tam, H. Shao, Nat. Nanotechnol. 16, 734 (2021)
R.S. Tade, P.O. Patil, A.C.S. Biomater, Sci. Eng. 6, 5987 (2020)
H. Chen, W. Zhang, G. Zhu, J. **e, X. Chen, Nat. Rev. Mater. 2, 17024 (2017)
D. Liu, Z. Zhou, X. Wang, H. Deng, L. Sun, H. Lin, F. Kang, Y. Zhang, Z. Wang, W. Yang, Biomaterials 244, 119979 (2020)
V. Bitonto, D. Alberti, R. Ruiu, S. Aime, S.G. Crich, J.C. Cutrin, J. Control. Release 319, 300 (2020)
N. Dammes, D. Peer, Theranostics 10, 938 (2020)
S. Urnauer, K.A. Schmohl, M. Tutter, C. Schug, N. Schwenk, S. Morys, S. Ziegler, P. Bartenstein, D.-A. Clevert, E. Wagner, Gene Ther. 26, 93 (2019)
Q. Liu, J. Tian, Y. Tian, Q. Sun, D. Sun, F. Wang, H. Xu, G. Ying, J. Wang, A.K. Yetisen, N. Jiang, ACS Nano 15, 515 (2021)
L. Golubewa, I. Timoshchenko, O. Romanov, R. Karpicz, T. Kulahava, D. Rutkauskas, M. Shuba, A. Dementjev, Y. Svirko, P. Kuzhir, Sci. Rep. 10, 22174 (2020)
L. Deng, H. Liang, S. Fu, R.R. Weichselbaum, Y.-X. Fu, Clin. Cancer Res. 22, 20 (2016)
C.M. Washington, D.T. Leaver, Principles and Practice of Radiation Therapy-E-Book (Elsevier Health Sciences, 2015)
M. Baumann, M. Krause, J. Overgaard, J. Debus, S.M. Bentzen, J. Daartz, C. Richter, D. Zips, T. Bortfeld, Nat. Rev. Cancer 16, 234 (2016)
H.-C. Chi, C.-Y. Tsai, M.-M. Tsai, C.-T. Yeh, K.-H. Lin, Int. J. Mol. Sci. 18, 1903 (2017)
J. Choi, G. Kim, S.B. Cho, H.-J. Im, J. Nanobiotechnology 18, 122 (2020)
W.N. Rahman, N. Bishara, T. Ackerly, C.F. He, P. Jackson, C. Wong, R. Davidson, M. Geso, Nanomed. Nanotechnol. Biol. Med. 5, 136 (2009)
F. Lux, V.L. Tran, E. Thomas, S. Dufort, F. Rossetti, M. Martini, C. Truillet, T. Doussineau, G. Bort, F. Denat, Br. J. Radiol. 92, 20180365 (2019)
A. Mignot, C. Truillet, F. Lux, L. Sancey, C. Louis, F. Denat, F. Boschetti, L. Bocher, A. Gloter, O. Stéphan, Chem. A Eur. J. 19, 6122 (2013)
Y. Du, H. Sun, F. Lux, Y. **e, L. Du, C. Xu, H. Zhang, N. He, J. Wang, Y. Liu, A.C.S. Appl, Mater. Interfaces 12(51), 56874–56885 (2020)
W. Sun, L. Luo, Y. Feng, Y. Qiu, C. Shi, S. Meng, X. Chen, H. Chen, Adv. Mater. 32, 2000377 (2020)
M.R.K. Ali, Y. Wu, M.A. El-Sayed, J. Phys. Chem. C 123, 15375 (2019)
S.C. Gad, K.L. Sharp, C. Montgomery, J.D. Payne, G.P. Goodrich, Int. J. Toxicol. 31, 584 (2012)
S. Bonvalot, C. Le Pechoux, T. De Baere, G. Kantor, X. Buy, E. Stoeckle, P. Terrier, P. Sargos, J.M. Coindre, N. Lassau, Clin. Cancer Res. 23, 908 (2017)
S. Bonvalot, P.L. Rutkowski, J. Thariat, S. Carrère, A. Ducassou, M.-P. Sunyach, P. Agoston, A. Hong, A. Mervoyer, M. Rastrelli, Lancet Oncol. 20, 1148 (2019)
D.A. Toesca, B. Ibragimov, A.J. Koong, L. **ng, A.C. Koong, D.T. Chang, J. Radiat. Res. 59, i40 (2018)
J. Jung, S.M. Yoon, S.Y. Kim, B. Cho, J.-H. Park, S.S. Kim, S.Y. Song, S.-W. Lee, S. Do Ahn, E.K. Choi, Radiat. Oncol. 8, 1 (2013)
C.-E. Hsieh, B.P. Venkatesulu, C.-H. Lee, S.-P. Hung, P.-F. Wong, S.P. Aithala, B.K. Kim, A. Rao, J.T.-C. Chang, N.-M. Tsang, Int. J. Radiat. Oncol. Biol. Phys. 105, 73 (2019)
J.S. Witt, S.A. Rosenberg, M.F. Bassetti, Lancet Oncol. 21, e74 (2020)
E.B. Golden, L. Apetoh, Seminars in Radiation Oncology (Elsevier, 2015), p. 11
J. Guo, Z. Yu, D. Sun, Y. Zou, Y. Liu, L. Huang, Mol. Cancer 20, 1 (2021)
A. Garg, S. Martin, J. Golab, P. Agostinis, Cell Death Differ. 21, 26 (2014)
T.B. Dar, R.M. Henson, S.L. Shiao, Front. Immunol. 9, 3077 (2019)
G. Song, L. Cheng, Y. Chao, K. Yang, Z. Liu, Adv. Mater. 29, 1700996 (2017)
N. Yang, W. **ao, X. Song, W. Wang, X. Dong, Nano-Micro Lett. 12, 1 (2020)
I.S. Harris, G.M. DeNicola, Trends Cell Biol. 30(6), 440–451 (2020)
Y. Liu, J. Zhang, J. Du, K. Song, J. Liu, X. Wang, B. Li, R. Ouyang, Y. Miao, Y. Sun, Acta Biomater. 129, 280 (2021)
Z. Huang, Y. Wang, D. Yao, J. Wu, Y. Hu, A. Yuan, Nat. Commun. 12, 145 (2021)
M. Heckler, N. Osterberg, J. Guenzle, N.K. Thiede-Stan, W. Reichardt, C. Weidensteiner, J.E. Saavedra, A. Weyerbrock, Tumor Biol. 39, 1010428317703922 (2017)
L. Gong, Y. Zhang, C. Liu, M. Zhang, S. Han, Int. J. Nanomedicine 16, 1083 (2021)
W. Tang, Z. Yang, L. He, L. Deng, P. Fathi, S. Zhu, L. Li, B. Shen, Z. Wang, O. Jacobson, J. Song, J. Zou, P. Hu, M. Wang, J. Mu, Y. Cheng, Y. Ma, L. Tang, W. Fan, X. Chen, Nat. Commun. 12, 523 (2021)
J. Thariat, J. Hérault, A. Beddok, L. Feuvret, D. Dauvergne, M. Gérard, J. Balosso, G. Noël, S. Valable, Cancer/Radiothérapie 24, 429 (2020)
H. Willers, A. Allen, D. Grosshans, S.J. McMahon, C. von Neubeck, C. Wiese, B. Vikram, Radiother. Oncol. 128, 68 (2018)
K. Konings, C. Vandevoorde, B. Baselet, S. Baatout, M. Moreels, Front. Oncol. 10, 128 (2020)
G. Bort, F. Lux, S. Dufort, Y. Crémillieux, C. Verry, O. Tillement, Theranostics 10, 1319 (2020)
P. Kowalik, J. Mikulski, A. Borodziuk, M. Duda, I. Kamińska, K. Zajdel, J. Rybusinski, J. Szczytko, T. Wojciechowski, K. Sobczak, R. Minikayev, M. Kulpa-Greszta, R. Pazik, P. Grzaczkowska, K. Fronc, M. Lapinski, M. Frontczak-Baniewicz, B. Sikora, J. Phys. Chem. C 124, 6871 (2020)
K. Deh, M. Zaman, Y. Vedvyas, Z. Liu, K.M. Gillen, P. O’Malley, D. Bedretdinova, T. Nguyen, R. Lee, P. Spincemaille, J. Kim, Y. Wang, M.M. **, Sci. Rep. 10, 1171 (2020)
C. Koo, H. Hong, P.W. Im, H. Kim, C. Lee, X. **, B. Yan, W. Lee, H.-J. Im, S.H. Paek, Nano Converg. 7, 1 (2020)
K. Simeonidis, C. Martinez-Boubeta, D. Serantes, S. Ruta, O. Chubykalo-Fesenko, R. Chantrell, J. Oró-Solé, L. Balcells, A.S. Kamzin, R.A. Nazipov, A. Makridis, M. Angelakeris, ACS Appl. Nano Mater. 3, 4465 (2020)
V. Mulens-Arias, A. Nicolás-Boluda, A. Pinto, A. Balfourier, F. Carn, A.K. Silva, M. Pocard, F. Gazeau, ACS Nano 15, 3330 (2021)
S. Samadzadeh, M. Babazadeh, N. Zarghami, Y. Pilehvar-Soltanahmadi, H. Mousazadeh, Mater. Sci. Eng. C 118, 111384 (2021)
J. Gao, F. Wang, S. Wang, L. Liu, K. Liu, Y. Ye, Z. Wang, H. Wang, B. Chen, J. Jiang, Adv. 7, 1903642 (2020)
D. Liu, Y. Hong, Y. Li, C. Hu, T.-C. Yip, W.-K. Yu, Y. Zhu, C.-C. Fong, W. Wang, S.-K. Au, Theranostics 10, 1181 (2020)
Y. Jiang, J. Huang, C. Xu, K. Pu, Nat. Commun. 12, 742 (2021)
Z. Yang, N. Zhao, J. Cui, H. Wu, J. **ong, T. Peng, Cell. Oncol. 43, 123 (2020)
Y. Yu, X. Yang, S. Reghu, S.C. Kaul, R. Wadhwa, E. Miyako, Nat. Commun. 11, 4117 (2020)
Z. Zhou, X. Wang, H. Zhang, H. Huang, L. Sun, L. Ma, Y. Du, C. Pei, Q. Zhang, H. Li, Small 17, 2007486 (2021)
Y Chen, M Gao, L Zhang, E Ha, X Hu, R Zou, L Yan, J Hu, Adv. Healthcare Mater. 2001665 (2020).
K. Hu, L. **e, Y. Zhang, M. Hanyu, Z. Yang, K. Nagatsu, H. Suzuki, J. Ouyang, X. Ji, J. Wei, Nat. Commun. 11, 1 (2020)
S. Lin, T. Yu, Z. Yu, X. Hu, D. Yin, Adv. Mater. 30, 1705691 (2018)
R. Bawa, Curr. Drug Del. 8, 227 (2011)
S. Mühlebach, Adv. Drug Del. Rev. 131, 122 (2018)
I.P. Kaur, V. Kakkar, P.K. Deol, M. Yadav, M. Singh, I. Sharma, J. Control. Release 193, 51 (2014)
M.K. Wilson, M.L. Friedlander, F. Joly, A.M. Oza, Oncologist 23, 203 (2018)
D U Ekwueme, K R Yabroff, G P Guy, Jr., M P Banegas, J S de Moor, C Li, X Han, Z Zheng, A Soni, A Davidoff, R Rechis, K S Virgo, C Centers for Disease, Prevention, MMWR. Morbidity and mortality weekly report 63, 505 (2014)
J. W. Weis, J. M. Jacobson, Nat. Biotechnol. 39, 1300 (2021)
O. Adir, M. Poley, G. Chen, S. Froim, N. Krinsky, J. Shklover, J. Shainsky-Roitman, T. Lammers, A. Schroeder, Adv. Mater. 32, 1901989 (2020)
W. Poon, B.R. Kingston, B. Ouyang, W. Ngo, W.C. Chan, Nat. Nanotechnol. 15, 819 (2020)
M. Alafeef, I. Srivastava, D. Pan, ACS Sens. 5, 1689 (2020)
O. Oktay, J. Nanavati, A. Schwaighofer, D. Carter, M. Bristow, R. Tanno, R. Jena, G. Barnett, D. Noble, Y. Rimmer, JAMA Netw Open 3, e2027426 (2020)
M. Cui, D.Y. Zhang, Lab. Invest. 101, 412 (2021)
J. Li, H. Chen, Y. Wang, M.-J.M. Chen, H. Liang, Cancer Cell 39, 3 (2021)
E. Calabrese, J.E. Villanueva-Meyer, S. Cha, Sci. Rep. 10, 1 (2020)
Acknowledgements
None.
Funding
This work was financially supported by an NIH R21 grant (1R21CA228099-01A1).
Author information
Authors and Affiliations
Contributions
JAK and YJK conceived the theme and created contents of the paper. JAK drafted the manuscript under the direction of YJK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kemp, J.A., Kwon, Y.J. Cancer nanotechnology: current status and perspectives. Nano Convergence 8, 34 (2021). https://doi.org/10.1186/s40580-021-00282-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40580-021-00282-7