Abstract
Background
Toxoplasma gondii and Neospora caninum are important protozoan parasites with worldwide distribution among warm-blooded animals. Moreover, T. gondii is a zoonotic parasite that infects humans. Poultries are important intermediated hosts of T. gondii and N. caninum. However, little is known about the contamination of poultry eggs with these parasites. We aimed to investigate the molecular frequency of T. gondii and N. caninum among the eggs of chicken, domestic duck, and quail from three different geographic regions of Iran. T. gondii and N. caninum were detected by PCR targeting the RE and Nc5 genes, respectively.
Findings
Overall contamination rates with T. gondii and N. caninum were 10.7 and 5.9%, respectively. The overall contamination rates of T. gondii among chicken, duck, and quail were 12.2, 15.5, and 4.4%, respectively; while N. caninum was detected in 11.1, 3.3, and 1.1% of the same samples, respectively. The contamination rates were increased with increasing humidity across three different regions.
Conclusions
Taken together, this study indicates the contamination of poultry eggs with T. gondii and N. caninum. The possibility of egg-born transmission of T. gondii should not be neglected by consuming raw soft-boiled eggs. Furthermore, contamination of poultry eggs could be an indicator for environmental contamination by these parasites.
Similar content being viewed by others
Introduction
Toxoplasma gondii and Neospora caninum are two closely related cyst-forming protozoan parasites with worldwide distribution among warm-blooded animals (de Barros et al. 2018; Dubey and Schares 2011a; Frenkel and Smith 2003a; Schlüter et al. 2014). Poultries are among the most important intermediate hosts for T. gondii (Dubey 2002; Dubey et al. 2020) and N. caninum (de Barros et al. 2018; Dubey and Schares 2011a). However, little is known about contamination poultry eggs by T. gondii and N. caninum. The objective of this study was to investigate the molecular detection of T. gondii and N. caninum in eggs of chicken (Gallus domesticus), domestic ducks (Anas platyrhynchos), and quails (Coturnix coturnix) from three different geographic regions of Iran.
Results
As shown in Table 1, T. gondii and N. caninum were detected in 29 (10.7%) and 16 (5.9%) out of 270 samples. Regarding the sampling site, the total frequency of T. gondii and N. caninum DNAs were higher in Astara (16.6 and 7.7%, respectively) than Kermanshah (11.1 and 5.5%) and Jahrom (4.4 and 3.3%). Regarding the poultry species, the total frequencies of T. gondii among chicken, duck, and quail were 12.2, 15.5, and 4.4%, respectively; while, the total frequencies of N. caninum were 11.1, 3.3, and 1.1%, respectively (Table 1). We did not detect any co-contamination of T. gondii and N. caninum in the samples.
Discussion
Both T. gondii and N. caninum are important protozoan parasites that infect a wide variety of warm-blooded animals, including bird species. However, T. gondii is more important because of their zoonotic potential for human health. Poultries could be an important reservoir of these parasites for their final host (Dubey and Schares 2011b; Schlüter et al. 2014). Indeed, humans can become infected by T. gondii by consuming undercooked avian tissues, including chickens and ducks (Dubey 2010; Dubey et al. 2020). Experimental studies revealed that chicken embryonated eggs could be an experimental model for T. gondii (Setasimy and Namavari 2016; Zuckerman 1966) and N. caninum (Furuta et al. 2007; Mansourian et al. 2009). However, in earlier reports with parasitological methods, only a few eggs had viable T. gondii following experimental infection of hens (Biancifiori et al. 1986; Boch et al. 1966; Dubey 2010; Jacobs and Melton 1966; Sokolov 1970), and there are no available data regarding N. caninum in this regard. T. gondii has been isolated from different organs of naturally infected hens, including ovaries and oviducts (Foster et al. 1969; Jacobs and Melton 1966); hence, the route of egg contamination may originate from ovaries and oviducts. Although we only detected the parasite DNA in avian eggs and did not perform methods for isolation of viable parasites (e.g., mouse bioassay), the possibility of egg-born transmission of these parasites should not be neglected. Contamination of avian eggs could also be an indicator for their host infection by N. caninum and T. gondii. Hence, detection of these parasites in avian eggs might be an alternative method for assessment of infection in their host. Inasmuch as the parasite oocysts are prevalent in the environment (Maleki et al. 2021), poultry could get the infection be feeding in the contaminated environment, therefore, infection of poultry might represent environmental contamination with the parasite’s oocyst. Likewise, the feline and canine definitive host of the parasites could get the infection by eating the infected intermediated hosts of the parasites.
In the current study, the total frequencies of T. gondii and N. caninum were 10.7 and 5.9% in three geographic regions of Iran. In our previous study, we detected T. gondii DNA by PCR method among 11% of chicken eggs in the south of Iran (Khademi et al. 2018). Both T. gondii and N. caninum have been detected among chickens and ducks in a number of studies (de Barros et al. 2018; Dubey 2010; Dubey and Schares 2011a; Dubey et al. 2020). In our study, contamination with T. gondii was detected in 12.2, 15.5, and 4.4% of chicken, duck, and quail, respectively. Studies indicate that chickens are resistant to toxoplasmosis, but there are few reports on confirmed clinical toxoplasmosis in chickens (Reviewed by (Dubey 2010)). Among the poultry species, quail eggs had the lowest contamination rate of both T. gondii and N. caninum in our study (4.4% vs. 1.1%, respectively). In two previous studies in China, the molecular frequency and seroprevalence rates of T. gondii among quails that intended for human consumption were reported to be 6.41% (Cong et al. 2017b) and 9.52% (Cong et al. 2017a), respectively. However, we only detected N. caninum DNA in one (1.1%) out of 90 quail eggs. Data regarding the prevalence of N. caninum among quails are scarce; nevertheless, an experimental study revealed that quails are resistant to infection with N. caninum (Oliveira et al. 2013).
Climate conditions are important factors for maintaining the parasite oocysts in the environment. While a relatively mild climate with humid conditions provides a suitable circumstance for oocysts to be active longer in the soil (Maleki et al. 2021). Hence, exposure and infection of animals in the regions with humid conditions are increased. Consequently, the biological transmission rates of these parasites could be increased in this condition. The higher prevalence of T. gondii and N. caninum in Astara County probably related to the mild temperature and higher humidity of this region than the two other sampling sites. A relatively mild temperature and higher humidity favor the survival and sporulation of coccidian oocysts, resulting in an increased risk of infection in human and animals (Dubey et al. 2007). The results of the current study have shown that the overall prevalence of T. gondii and N. caninum was higher in Astara county (which has a humid subtropical climate with an average relative humidity of 82%) than Kermanshah province (which has a Mediterranean climate with an average relative humidity of 49%) and Jahrom county (which has a hot semi-arid climate with an average relative humidity of 46%). Our recent meta-analysis revealed that increasing relative humidity (≥76%) is associated with an increasing trend in the prevalence of T. gondii oocysts in the soil of public places (Maleki et al. 2021). In our previous study (Khademi et al. 2021), we assessed the molecular prevalence of T. gondii in slaughtered sheep in two high- and low-humidity regions in the south of Iran (Hormozgan Province). The results revealed that the rate of T. gondii was 10.7% of the samples from the highly humid region, while no positive samples were detected in the low-humidity region. Our another investigation in the same region of Iran was performed the molecular detection of T. gondii in chicken eggs (Khademi et al. 2018). The results demonstrated that T. gondii was positive among 11% of the chicken eggs in the area with hot and humid weather; whereas, no T. gondii was detected in the chicken eggs in the area with warm and dry weather (Khademi et al. 2018). Moreover, our recent reports among pregnant women in the same region of Iran (Hormozgan Province) revealed that pregnant women are at high risk of congenital toxoplasmosis (Khademi et al. 2019; Khademi et al. 2022).
Conclusions
Our study indicates contamination of chickens, ducks, and quails with T. gondii and N. caninum in three different geographic regions of Iran. Our other findings revealed that increasing humidity was associated with increased prevalence of T. gondii and N. caninum contamination in the eggs of chickens, ducks, and quails. Although we did not perform methods for detection of viable T. gondii and N. caninum (e.g., mouse bioassay), the possibility of egg-born transmission of T. gondii should not be neglected by consuming raw soft-boiled eggs. Moreover, contamination of poultry eggs could be an indicator for environmental contamination by these parasites.
Materials and methods
Study area and sample collection
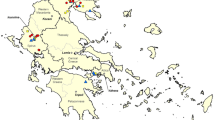
The samples were purchased from local markets in three different geographic regions of Iran (Fig. 1), including Astara county (north of Iran, border of Caspian Sea), Kermanshah province (western Iran), and Jahrom (nearly south of Iran). We selected two local markets in each region. We selected those local markets that sold traditional and local foods. The eggs came from small farms in rural areas to the markets for selling. A total of 270 eggs of chicken, quail, and duck (30 eggs from each bird from each city) was purchased during the spring and summer. Figure 1 represents the climate map of Iran and the climate conditions of each sampling site.
The climate map of Iran. https://commons.wikimedia.org/w/index.php?title=File:Iran-climate.png&oldid=224111575. The samples were taken from Astara (north of Iran, border of Caspian Sea), Kermanshah province in western Iran, and Jahrom in nearly south of Iran. Astara: Coordinates: 38°25′45″N 48°52′19″E. Astara county has a humid subtropical climate with relatively cold, wet winters and hot, humid summers. This county has an average rainfall of 1292.5 mm per year, average rainy days (≥ 1.0 mm) of 112 days, and average relative humidity (%) of 82% (https://en.wikipedia.org/wiki/Astara,_Iran. Kermanshah province: Coordinates: 34°18′51″N 47°03′54″E. Kermanshah classified as Mediterranean climate, experiences rather cold winters and a hot-summer. Kermanshah has an average rainfall of 478.7 mm per year, average rainy days (≥ 1.0 mm) of 77.3 days, and average relative humidity (%) of 49% (https://en.wikipedia.org/wiki/Kermanshah). Jahrom: Coordinates: 28°30′00″N 53°33′38″E. Jahrom county has a hot semi-arid climate. The average rainfall is about 285 mm (11.2 in) per year. The average rainy days (≥ 1.0 mm) of 27 days, and average relative humidity (%) of 46% (https://en.wikipedia.org/wiki/Jahrom)
Sample preparation, DNA extraction, and PCR
The egg shells were washed individually before separation of the albumen and yolk. Then, albumen and yolk of each egg were completely mixed (Khademi et al. 2018) and DNA extraction was performed using the phenol–chloroform extraction method as described (Abdoli et al. 2015). Tachyzoites of the RH and Nc5 strains of T. gondii and N. caninum were used as positive control for DNA extraction. Molecular detection of T. gondii was performed by primers targeting the repetitive element (RE) gene (Homan et al. 2000) as described in our previous reports (Abdoli et al. 2016; Khademi et al. 2018; Khademi et al. 2021; Rasti et al. 2018). Molecular detection of N. caninum was performed by nested-PCR method using specific primers that amplified the Nc5 gene (Hughes et al. 2006) as described in our previous reports (Abdoli et al. 2015; Arbabi et al. 2016). The primer sequences and PCR cycling conditions are presented in Supplementary Tables 1 and 2. The DNAs were amplified in a 20 μl reaction mixture containing 3 μL of template DNA, 10 μl of commercial master mix containing 2x Taq DNA polymerase with 1.5 mM MgCl2 (Cat. no. A170301, Ampliqon, Denmark), 5 μL of distilled water, and 10 pmol of each primer. For nested PCR of the NC5 gene, the same conditions were performed, except for the use of 25 pmol of primers for nested-PCR reaction (Hughes et al. 2006) and using one μL of one-tenth diluted of the first round PCR product as the template for nested-PCR. In each reaction, a positive control and a negative control (double distilled water) were included. PCR products were analyzed on 2% agarose gels stained with Safe stain (Sinaclon, Iran) that electrophoresed in Tris-acetate-EDTA (TAE) buffer and evaluated under ultraviolet transillumination.
Availability of data and materials
Not applicable.
Change history
27 April 2023
A Correction to this paper has been published: https://doi.org/10.1186/s40550-023-00105-z
References
Abdoli A, Arbabi M, Dalimi A, Pirestani M (2015) Molecular detection of Neospora caninum in house sparrows (Passer domesticus) in Iran. Avian Pathol 44:4, 319-322
Abdoli A, Dalimi A, Soltanghoraee H, Ghaffarifar F (2016) Molecular detection of Toxoplasma gondii in house sparrow (Passer domesticus) by LAMP and PCR methods in Tehran, Iran. J Parasitic Dis 40:1317–1321
Arbabi M, Abdoli A, Dalimi A, Pirestani M (2016) Identification of latent neosporosis in sheep in Tehran, Iran by polymerase chain reaction using primers specific for the Nc-5 gene. Onderstepoort J VetRes 83:1–7
Biancifiori F, Rondini C, Grelloni V, Frescura T (1986) Avian toxoplasmosis: experimental infection of chicken and pigeon. Comp Immunol Microbiol Infect Dis 9:337–346
Boch J, Rommel M, Weiland G, Janitschke K, Sommer R (1966) Experimentelle Toxoplasma-Infektionen bei Legehennen. Berl Munch Tierarztl Wochenschr 79:352–355
Cong W, Chi W-B, Sun W-W, Shan X-F, Kang Y-H, Meng Q-F, Qian A-D (2017a) First report of toxoplasma gondii infection in common quails (Coturnix coturnix) intended for human consumption in three provinces of northeastern China. Vector-Borne and Zoonotic Diseases 17:351–353
Cong W, Ju H-L, Zhang X-X, Meng Q-F, Ma J-G, Qian A-D, Zhu X-Q (2017b) First genetic characterization of Toxoplasma gondii infection in common quails (Coturnix coturnix) intended for human consumption in China. Infect Genet Evol 49:14–16
de Barros LD, Miura AC, Minutti AF, Vidotto O, Garcia JL (2018) Neospora caninum in birds: a review. Parasitol Int 67:397–402
Dubey J (2002) A review of toxoplasmosis in wild birds. Vet Parasitol 106:121–153
Dubey J (2010) Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 57:60–73
Dubey J, Schares G (2011a) Neosporosis in animals—the last five years. Vet Parasitol 180:90–108
Dubey J, Schares G, Ortega-Mora L (2007) Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 20:323–367
Dubey JP, Pena HFJ, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Yang YR, Gennari SM, Su C (2020) Epidemiologic significance of Toxoplasma gondii infections in chickens (Gallus domesticus): the past decade. Parasitology 147:1263–1289
Dubey JP, Schares G (2011b) Neosporosis in animals—the last five years. Vet Parasitol 180:90–108
Foster B, Forrest R, Blanco J (1969) Isolation of Toxoplasma gondii from naturally infected chickens. Tex J Sci 20:323–328
Frenkel J, Smith D (2003a) Determination of the genera of cyst-forming coccidia. Parasitol Res 91:384–389
Furuta PI, Mineo TWP, Carrasco AOT, Godoy GS, Pinto AA, Machado RZ (2007) Neospora caninum infection in birds: experimental infections in chicken and embryonated eggs. Parasitology 134:1931–1939
Homan WL, Vercammen M, De Braekeleer J, Verschueren H (2000) Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol 30:69–75
Hughes J, Williams R, Morley E, Cook D, Terry R, Murphy R, Smith J, Hide G (2006) The prevalence of Neospora caninum and co-infection with Toxoplasma gondii by PCR analysis in naturally occurring mammal populations. Parasitology 132:29–36
Jacobs L, Melton ML (1966) Toxoplasmosis in chickens. J Parasitol 52:1158–1162
Khademi SZ, Ghaffarifar F, Dalimi A, Davoodian P, Abdoli A (2018) Molecular detection and genotype identification of Toxoplasma gondii in domestic and industrial eggs. J Food Saf 38:e12534
Khademi SZ, Ghaffarifar F, Dalimi A, Davoodian P, Abdoli A (2019) Prevalence and risk factors of Toxoplasma gondii infection among pregnant women in Hormozgan Province, South of Iran. Iran J Parasitol 14:167
Khademi SZ, Ghaffarifar F, Dalimi A, Davoodian P, Abdoli A, (2022) Spontaneous abortion among Toxoplasma gondii IgG seropositive women: molecular detection, genotype identification, and serological assessment with conventional ELISA and avidity ELISA. J Obstet Gynaecol Res in press https://doi.org/10.1111/jog.15349
Khademi SZ, Ghaffarifar F, Dalimi A, Dayer MS, Abdoli A (2021) Toxoplasma gondii in slaughtered sheep in high- and low-humidity regions in the south of Iran: molecular prevalence and genotype identification. Vet Med Int 2021:5576771
Maleki B, Ahmadi N, Olfatifar M, Gorgipour M, Taghipour A, Abdoli A, Khorshidi A, Foroutan M, Mirzapour A (2021) Toxoplasma oocysts in the soil of public places worldwide: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 115:471–481
Mansourian M, Khodakaram-Tafti A, Namavari M (2009) Histopathological and clinical investigations in Neospora caninum experimentally infected broiler chicken embryonated eggs. Vet Parasitol 166:185–190
Oliveira UVD, de Magalhães VCS, Almeida CP, Santos IDA, Mota DA, Macêdo LS, Silva FL, Carvalho FDS, Wenceslau AA, Munhoz AD (2013) Quails are resistant to infection with Neospora caninum tachyzoites. Vet Parasitol 198:209–213
Rasti S, Marandi N, Abdoli A, Delavari M, Mousavi SGA (2018) Serological and molecular detection of Toxoplasma gondii in sheep and goats in Kashan, Central Iran. J Food Saf 38:e12425
Schlüter D, Däubener W, Schares G, Groß U, Pleyer U, Lüder C (2014) Animals are key to human toxoplasmosis. Int J Med Microbiol 304:917–929
Setasimy A, Namavari M (2016) Use of chicken embryonated eggs for evaluating the virulence of Toxoplasma gondii. J Parasit Dis 40:1223–1225
Sokolov A (1970) Susceptibility of chickens to Toxoplasma gondii. Veterinariia 5:79–80
Zuckerman A (1966) Propagation of parasitic protozoa in tissue culture and avian embryos. Ann N Y Acad Sci 139:24–38
Acknowledgments
The authors acknowledge the Jahrom University of Medical Sciences, Jahrom, Iran, for supporting this study. The authors also would like to thank the Clinical Research Development Unit of Peymanieh Educational and Research and Therapeutic Center of the Jahrom University of Medical Sciences for supporting this work.
Funding
This study was supported by the Jahrom University of Medical Sciences, Jahrom, Iran (grant NO: 98001491).
Author information
Authors and Affiliations
Contributions
AA conceived the study. SM, MAB, AB, HGB, and FB were obtained the samples. HM, and AA performed laboratory tests. KS, SZK, MS and AA analyzed the results, designed the tables and fig. AA wrote the draft of the manuscript. AA and FG supervised the study. All authors approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the research and ethics committee of the Jahrom University of Medical Sciences, Jahrom, Iran (the ethics code: IR.JUMS.REC.1398.061).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
T. gondii and N. caninum specific primers targeting the RE and Nc5 genes. Supplementary Table 2. PCR conditions of the Nc5 and RE genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maani, S., Solhjoo, K., Bagherzadeh, M.A. et al. Prevalence of Toxoplasma gondii and Neospora caninum contaminations in poultry eggs: molecular surveillance in three different geographical regions of Iran. Food saf. and Risk 10, 3 (2023). https://doi.org/10.1186/s40550-023-00103-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40550-023-00103-1