Abstract
Neonicotinoid insecticides have raised a lot of societal concerns due to their environmental ubiquity and unique mode of action. Therefore, it is of great research interest to monitor their occurrence in the environmental waters. However, these compounds exist at low concentrations that is below instrument detection limits. This study reports the applicability of magnetic poly (3 aminobenzoic acid)-based activated carbon (Fe3O4@PABA/AC) composite as an adsorbent in dispersive magnetic solid-phase microextraction (d-MSPME) of neonicotinoid insecticides from wastewater and river water samples. The as-synthesized adsorbent was characterized and confirmed by Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, Brunauer–Emmett–Teller and X-ray diffraction spectroscopy. The analytes of interest were detected and quantified by high-performance liquid chromatography coupled with diode array detector (HPLC–DAD). The parameters affecting the extraction and preconcentration processes, such as pH, extraction time, mass of adsorbent, desorption time and eluent volume, were optimized using fractional factorial design and central composite design. Under optimum conditions, the limits of detection and quantification were in the ranges of 0.41–0.82 µg L−1 and 1.4–2.7 µg L−1, respectively. The linearity ranged from 1.4–700 µg L−1 with correlation of determination (R2) values varied between 0.9933 and 0.9987. The intra-day and inter-day precisions were 0.35–0.75% and 1.7–5.5%, respectively. The spike recovery experiments were conducted to evaluate the accuracy of the d-MSPME analytical method in real samples, and the percentage recoveries ranged from 86.7 to 99.2%. Therefore, this method shows great potential applicability in preconcentrating the pollutants from the environment.
Similar content being viewed by others
Introduction
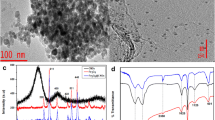
Recently, access to clean water has become a global concern. The remarkable increase in human population has triggered high industrialization and agricultural production to meet mankind sustainability (Munonde 2017). These activities lead to increased use of various chemical substances that are then released into the environment. Among these chemical substances, neonicotinoid insecticides (also known as neonics) are the mostly distributed into the environment via agricultural practices (Vichapong et al. 2015). Neonicotinoid insecticides are a relatively new class of insecticides derived from nicotine, and they were introduced in the 1990s for insects control (Yáñez et al. 2014; Farajzadeh et al. 2016). They act agonistically at the nicotinic acetylcholine receptors (nAChRs) on the post-synaptic layer. These receptors contribute greatly to signal transmission in the central nervous system (CNS) (Vichapong et al. 2013; Jovanov et al. 2013; Zhao et al. 2020; Guo et al. 2020; Yamamoto and Casida 1999). Neonicotinoids bind tightly to the nAChRs of insects than to any other organism including mammals, and the latter can cause paralysis or even death. However, these compounds are considered less toxic to mammals (Farajzadeh et al. 2016; Zou et al. 2018). The FTIR spectrum of the AC exhibited a broad peak around 3100 cm−1 which was attributed to the vibration of the surface alcoholic OH or phenolic OH groups. The incorporation of PABA on AC matrix resulted on the upward shift of the peak that was observed in FTIR spectrum of AC to s 3500 cm−1 (Fig. 2a). The AC spectrum shows absorption peaks at 1627, 1550 and 1390 cm−1 indicating the stretching vibration of C=O, the skeletal vibration of C=C and C=OH vibration, respectively (Mashile et al. 2018a). Magnetic nanoparticles spectrum showed the main intense asymmetrical peak at around 571 cm−1 corresponding to the Fe–O stretching vibrations (Tarhan et al. 2020). In Fig. 2b, the FTIR spectrum of PABA/AC exhibited a strong peak at 3448 cm−1 associated to the stretching vibration of O–H/N–H groups. The two small peaks at 2920 cm−1 and 2851 cm−1 represented the symmetric and asymmetric stretching of C–H bond (Awual et al. 2019; Naushad et al. 2019). The band at 1751 cm−1 was associated with the stretching vibration of C=O from carboxylic group. In addition, the amide groups stretching vibrations were observed at 1660 cm−1 showing the polymer bending on AC surface. The peaks at 1440 cm−1 and 1090 cm−1 indicated the stretching vibrations of N–H and C–N, respectively (Njoku et al. 2014), confirming the successful do** of PABA polymer on AC. The spectrum of Fe3O4@PABA/AC exhibited Fe–O stretching vibration peak at 571 cm−1 confirming the successful incorporation of magnetic nanoparticles in to the PABA/AC composite (Feng et al. 2020). Additionally, the disappearance of the peak at 1250 cm−1 was a sign of successful do** of iron oxide nanoparticles.
X-ray diffraction spectroscopy
The XRD patterns for magnetite (Fe3O4 nanoparticles), AC, PABA, PABA/AC and Fe3O4@PABA/AC nanocomposite are shown in Fig. 3. The XRD pattern of PABA exhibited a broad peak at 2θ angle of 26° which is an indication of the amorphous nature of PABA polymer, and it agreed with the recently reported XRD pattern of polyaniline (Mashao et al. 2019). Magnetic nanoparticles exhibited the main diffraction peaks at 2θ angle of 30.2, 35.4, 43.2, 53.6, 57.1, 62.9 and 74.3, which corresponded with (220), (311), (400), (422), (511), (440) and (533) hkl values, respectively. Additionally, these peaks matched with the reflections proposed by Bragg based on the spinel ferrite structure (Hammad et al. 2020). The broad peaks appeared at 2θ of 10°–30° and 30°–50° were the characteristic peaks of amorphous AC, matching (022) and (100) graphitic carbon diffraction pattern (Du et al. 2020; Wang et al. 2019). In the PABA/AC composite, the broad peak of PABA was still observed indicating that the PABA polymer sat on AC clusters. In the nanocomposite (Fe3O4@PABA/AC), it was observed that the magnetic nanoparticles were immobilized on the PABA/AC composite surface. Furthermore, some of the magnetite diffraction peaks were maintained but their intensities decreased proving the successful incorporation of magnetic nanoparticles onto the surface of PABA/AC. It should be noted that there are similarities in the XRD patterns of Fe3O4 and Fe3O4@PABA/AC, and this was expected because the crystallinity of magnetite influences the morphology of the composite. However, it is worth mentioning that the XRD patterns of Fe3O4@PABA/AC had reduced peak intensities compared to XRD spectrum of magnetite indicating a successful incorporation of Fe3O4 on PABA/AC surface.
Brunauer–Emmet–Teller
The surface area and pores of the synthesized materials were obtained using Brunauer–Emmett–Teller (BET) technique based on N2 adsorption–desorption, and the results are presented in Table 2.
It should be noted from Table 2 that the surface properties of the AC decreased dramatically after the incorporation of PABA. This could be due to the increase in the diameter of the PABA/AC which results in a decreased in surface area (Sadeghi et al. 2018). In addition, the lower amount of AC doped could also be the cause for surface properties decrease. A significant increase in surface area and pores was observed in the Fe3O4@PABA/AC composite after the incorporation of Fe3O4 nanoparticles. This could further confirm that Fe3O4 nanoparticles played a crucial role in neonicotinoids extraction by reducing the diameter of the PABA/AC composite, which in turn increases its surface area. The observed surface area and pores were enough for the maximum extraction of the neonicotinoids.
Scanning electron microscopy
Figure 4 shows the external morphologies and elemental composition of PABA/AC and Fe3O4@PABA/AC obtained using SEM/EDS. In the PABA/AC composite (Fig. 4a), PABA covered the surface of the porous AC. Furthermore, the AC clusters were minimized and were heterogeneously mixed with PABA matrix (Hasan et al. 2019). After the incorporation of Fe3O4 into the composite (Fig. 4c), pores were reduced. In addition, the coated Fe3O4 resulted in a smooth surface and reduced particles. The EDS spectrum (Fig. 4b) revealed the presence of oxygen and carbon in PABA/AC as the important elements of the nanocomposite. Chlorine and iron observed were from the oxidizing agent from PABA polymerization. After the incorporation of Fe3O4 (Fig. 4d), the weight percentage of iron increased drastically confirming the presence of Fe3O4 in the Fe3O4@PABA/AC composite.
Transmission electron microscopy
The internal morphological features of the synthesized materials were investigated using TEM analysis, and the images are shown in Fig. 5. The TEM image of PABA was similar to the previously reported image by Ramohlola et al. 2017a. In Fig. 5a, it should be observed that PABA sat on AC clusters. In the composite (Fig. 5b), Fe3O4 nanoparticles distributed dependently mostly on PABA surface owing to their affinity towards the groups that are abundant on the polymer. The agglomeration observed is caused by poor dispersibility of the Fe3O4 nanoparticles.
Optimization of the developed d-MSPME
Screening of d-MSPME preconcentration procedure using central composite design
To obtain best results from any analytical method, screening process was conducted to investigate the most influential parameters in the extraction and preconcentration of neonicotinoids. A two-level (25–1) FFD was employed as a chemometric tool for the screening of d-MSPME method. In this work, five parameters including pH, extraction time (ET), mass of adsorbent (MA), eluent volume (EV) and desorption time (DT) were optimized. Twenty experiments were conducted for screening and the results were recorded as percentage recoveries (Additional file 1: Table S1). The evaluation of the experimental data was done using analysis of variance (ANOVA), and the ANOVA results were recorded as Pareto charts (Fig. 6a–d). Pareto charts allowed the assessment of the main and interactive effects. The length of each bar in Fig. 6 represented the effects of individual factors and their interactions, and these bars are proportional to the effect of each factor or interactions. If the bar length surpasses the reference line, it implies that the factor or interaction has significant effect on the analytical response at 95% confidence level (Biata et al. 2017). As shown in Fig. 6a, b and d, mass of adsorbent and eluent volume were influential on analytical response for ACT, IMD and CLD, except for extraction and preconcentration of TCL where only EV was significant at 95% confidence level. Moreover, the interaction between MA and EV (2by3) was statistically significant. Ideally, the eluent volume must be sufficient to quantitatively extract the all the analytes from the surface of the adsorbent. In addition, enough adsorbent is required to adsorb the target analytes. Therefore, both MA and EV should be sufficient to achieve quantitative adsorption and elution of the analytes, respectively. The two factors (MA and EV) were further optimized using CCD to minimize the number of experiments and to investigate their interactions based on the quadratic model employed on response surface methodologies (RSM) (Li et al. 2019).
Pareto charts indicating the effect of factors on extraction and preconcentration of a acetamiprid (ACT), b imidacloprid (IMD), c thiacloprid (TCL) and d clothianidin (CLD) using magnetic-based PABA activated carbon adsorbent. ET extraction time, MA mass of adsorbent, EV eluent volume (EV) and DT desorption time. 1by2: interaction between pH and MA; 1by3: interaction between pH and EV; 1by4: interaction between pH and ET; 1by5: interaction between pH and DT; 2by3: interaction between MA and EV; 2by4: interaction between MA and ET; 2by5: interaction between MA and DT; 3by4: interaction between EV and ET; 3by5: interaction between EV and DT; 4by5: interaction between ET and DT
Further optimization
The preliminary studies indicated that the Fe3O4@PABA/AC was capable of adsorbing and desorbing the neonicotinoid insecticides. The factors that were affecting the performance of d-MSPME method were further investigated. These factors include MA and EV, and were optimized using CCD involving ten experiments, to evaluate their interacting effects with respect to the extraction recoveries (Additional file 1: Table S2). The interactive effects, main effects and quadratic effects towards the extraction of the neonicotinoids were investigated by the RSM based on CCD (Ferreira et al. 2019), and the results are presented as 3D surface plots in Fig. 7. The interactions between the factors were significant towards the extraction of IMD and CLD. Higher extraction recoveries were observed at higher MA and EV. However, the interactions of the factors towards the extraction of ACT and TLC did not show any statistical significance. The best precise conditions could not be assumed from the plots since the plots give the region not the precise points. Therefore, the desirability profiles were obtained by further optimization owing to their potential to pinpoint exact optimum values (Mashile et al. 2018b).
Figure 8 shows the desirability profiles of the thiacloprid obtained by the conversion of the responses into the dimensionless values called desirability by the application of statistical design. The aggregation of these individual desirability (di) are then utilized to obtain the overall desirability (Valasques et al. 2019; Bruns et al. 2006). According to the literature, desirability ranges from 0 (undesirable), 0.5 (partially desirable) and 1 (desirable) (Bezerra et al. 2019). Figure 8 also illustrates the individual desirability scores for the extraction and preconcentration of the target analytes (bottom), and the desirability value of 1.0 was selected to obtain optimum conditions. The percent recovery obtained from plots for each factor is presented at the top left-hand side. According to Mashile et al. 2018b, the figures on the top left-hand side demonstrate the changes in the level of each individual variable, analytical response, and the overall desirability. It can be seen from the desirability profile that both factors were considered significant since the lower points of these factors deviated from the desirable line indicating that a change in one of these values can cause dramatic effect on analytical responses (% extraction recoveries). The desirability profiles for the other three neonicotinoids showed similar results (Additional file 1: Fig. S1). The optimum conditions predicted from these desirability profiles and RSM were 41 mg for MA and 1200 µL for EV. The use of small MA for the extraction of the selected neonicotinoids in a short period of time has proved that the Fe3O4@PABA/AC adsorbent could be potentially applied in a wide range of sample preparation procedures including their miniaturized versions. Other parameters that were not significant in the screening process were kept constant at 10 min, 5 min and 6.5 for ET, DT, and pH, respectively.
Method validation
Under optimum conditions, the analytical performance of the developed d-MSPME procedure was assessed in terms of limits of detection (LOD), quantification (LOQ), linearity, repeatability and reproducibility (Table 3). The neonicotinoids standard solutions ranging from concentrations of 0–700 µg L−1 were preconcentrated using the d-MSPME and the calibration curve for each analyte was constructed. From the calibration curves, the linearity was from 1.4 to 700 µg L−1 with the correlation coefficients ranging from 0.9941 to 0.9987, respectively, which indicates good linearity. The LOD and LOQ were demonstrated based on the International Union of Pure and Applied Chemistry (IUPAC) as the lowest amounts of the analytes that can give responses equivalent to 3.0 or 10 times the original signals, respectively. They are expressed as the ratio of the standard deviation to the slope of the calibration curve (Mpupa et al. 2019; Komendova 2020). The LODs ranged from 0.41 to 0.82 µg L−1, while LOQs were between 1.4 and 2.7 µg L−1. The repeatability (intra-day) and reproducibility (inter-day) procedures were conducted to assess the precision of the method. These were expressed in terms of relative standard deviations (%RSD) based on ten replicates on the same day (repeatability, n = 10) and five different days (reproducibility, n = 5 working days). The intra-day precisions were found to be less than 1%, while the inter-day precisions were less than 6% (Table 3).
Adsorbent reusability
The attractive feature of an exceptional adsorbent includes high regeneration and reusability. This allows the reduction in the overall cost of the adsorption technology. Additionally, for economic reasons, the desorption of the analyte from an adsorbent is considered as significant approach to the reuse of spent adsorbents. In this study, the reusability studies were carried to determine the number of cycles a spent Fe3O4@PABA/AC nanocomposite can be used for the preconcentration of the neonicotinoids while maintaining maximum extraction recoveries. As seen in Additional file 1, Fig. S2, quantitative recoveries (≥ 80%) for all neonicotinoids were obtained when the adsorbent was used for at least 10 times with the %RSD ranging from 2.5 to 3.1%. Even though the adsorption efficiency decrease was observed, the spent adsorbent showed better stability and reusability. Therefore, it can be concluded that Fe3O4@PABA/AC nanocomposite had acceptable regeneration and reusability performances and can be viewed as a promising adsorbent for efficient extraction and preconcentration of various organic pollutants in water systems.
Application to real samples
The reliability of the d-MSPME method was conducted using the spike recovery tests. These tests were carried at two levels, 1.0 µg L−1 (lower level) and 5.0 µg L−1 (higher level) for all analytes (Table 4). It was clearly observed that the analytes of interest were not detected in all matrices (influent, effluent, and river water samples). The spiking process was done due to the absence of the certified reference material (CRM) for neonicotinoids. Each sample was analysed three times, and the response was obtained from the average of the outcomes. Maximum percentage recoveries (%R) were still obtained in the ranges of 86.7 and 99.2% for all analytes in all matrices, indicating that the matrices had no disadvantageous effects on extraction of the neonicotinoids. The relative standard deviation was < 5%, further proving that the effects from the matrix were very little for the proposed analytical procedure. The d-MSPME analytical method showed potential in extraction and preconcentration of trace neonicotinoids from various environmental wastewater matrices.
Comparison between the current proposed method and others
The performance of the current analytical method coupled with HPLC–DAD towards the preconcentration of neonicotinoids was compared with the methods reported recently. The parameters of the present proposed method and those of the previously reported methods are summarized in Table 5. The current proposed method had a good repeatability, and the relative standard deviation (RSDs) were smaller than those of the recently reported methods. Limits of detection of the presented method were lower than some of the mentioned methods and the linear ranges were reasonably wider. Compared to a relative method reported by Ghiasi et al. (2020) for the preconcentration of neonicotinoids, it can be concluded that the current method is facile, rapid, sensitive, and environmentally friendly towards the extraction and determination of neonicotinoids from various environmental samples. Furthermore, the method was able to provide maximum %R for four analytes in the presence of the interferences, and the %R was maintained even after ten successive cycles (Additional file 1: Fig. S2).
Conclusions
Magnetic-based PABA/AC nanocomposite was successfully synthesized and applied as an adsorbent in the extraction and preconcentration of the selected neonicotinoids (ACT, IMD, TCL and CLD) from model solutions and real water samples. The d-MSPME showed a great potential as a preconcentration method for the selected neonicotinoid insecticides. Fe3O4@PABA/AC nanocomposite was characterized using FTIR, SEM, TEM, BET and XRD. Before the application of the d-MSPME method in the real samples, parameters such as pH, ET, MA, DT and EV affecting the performance of the method were optimized for the extraction of neonicotinoids from spiked tap water. Under optimum conditions, the reliability of the d-MSPME method was evaluated based on LODs and LOQs, which ranged from 0.41 to 0.82 µg L−1 and 1.4 to 2.7 µg L−1, respectively. Fe3O4@PABA/AC showed high reusability which was confirmed by reusing the adsorbent at least 10 times while maintaining maximum recoveries. Furthermore, the adsorbent was used in the extraction of neonicotinoids from real water samples obtained from domestic WWTP. The adsorbent showed magnificent applicability in the neonicotinoids extraction, and this indicated that the nanocomposite might mitigate the global challenge based on monitoring trace organic pollutants in environmental wastewater.
Availability of data and materials
Not applicable.
References
Albishri HM, Marwani HM, Batterjee MG, Soliman EM. Eriochrome Blue Black modified activated carbon as solid phase extractor for removal of Pb (II) ions from water samples. Arab J Chem. 2017;10:S1955–62.
Anzecc A (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, pp 1–103.
Arase S, Horie K, Kato T, Noda A, Mito Y, Takahashi M, Yanagisawa T. Intelligent peak deconvolution through in-depth study of the data matrix from liquid chromatography coupled with a photo-diode array detector applied to pharmaceutical analysis. J Chromatogr A. 2016;1469:35–47.
Arnnok P, Patdhanagul N, Burakham R. Dispersive solid-phase extraction using polyaniline-modified zeolite NaY as a new sorbent for multiresidue analysis of pesticides in food and environmental samples. Talanta. 2017;164:651–61.
Awual MR, Hasan MM, Islam A, Rahman MM, Asiri AM, Khaleque MA, Sheikh MC. Introducing an amine functionalized novel conjugate material for toxic nitrite detection and adsorption from wastewater. J Clean Prod. 2019;228:778–85.
Azzouz A, Kailasa SK, Lee SS, Rascón AJ, Ballesteros E, Zhang M, Kim K-H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal Chem. 2018;108:347–69.
Bezerra MA, Ferreira SLC, Novaes CG, dos Santos AMP, Valasques GS, da Mata Cerqueira UMF, dos Santos Alves JP. Simultaneous optimization of multiple responses and its application in analytical chemistry—a review. Talanta. 2019;194:941–59.
Biata NR, Nyaba L, Ramontja J, Mketo N, Nomngongo PN. Determination of antimony and tin in beverages using inductively coupled plasma-optical emission spectrometry after ultrasound-assisted ionic liquid dispersive liquid–liquid phase microextraction. Food Chem. 2017;237:904–11.
Biswas A, Patra AK, Sarkar S, Das D, Chattopadhyay D, De S. Synthesis of highly magnetic iron oxide nanomaterials from waste iron by one-step approach. Colloids Surf A Physicochem Eng Asp. 2020;589:124420.
Bruns RE, Scarminio IS, de Barros Neto B. Statistical design-chemometrics, vol. 25. Amsterdam: Elsevier; 2006.
Chisvert A, Cárdenas S, Lucena R. Dispersive micro-solid phase extraction. TrAC Trends Anal Chem. 2019;112:226–33.
Dimpe KM, Ngila J, Nomngongo PN. Preparation and application of a tyre-based activated carbon solid phase extraction of heavy metals in wastewater samples. Phys Chem Earth Parts a/b/c. 2018;105:161–9.
Du H, Cheng J, Wang M, Tian M, Yang X, Wang Q. Red dye extracted sappan wood waste derived activated carbons characterization and dye adsorption properties. Diam Relat Mater. 2020;102:107.
Ebrahimi B, Mohammadiazar S, Ardalan S. New modified carbon based solid phase extraction sorbent prepared from wild cherry stone as natural raw material for the pre-concentration and determination of trace amounts of copper in food samples. Microchem J. 2019;147:666–73.
Farajzadeh MA, Bamorowat M, Mogaddam MRA. Ringer tablet-based ionic liquid phase microextraction: application in extraction and preconcentration of neonicotinoid insecticides from fruit juice and vegetable samples. Talanta. 2016;160:211–6.
Feng Z, Chen H, Li H, Yuan R, Wang F, Chen Z, Zhou B. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater. Sci Total Environ. 2020;713:136.
Ferreira SL, Junior MMS, Felix CS, da Silva DL, Santos AS, Neto JHS, de Souza CT, Junior RAC, Souza AS. Multivariate optimization techniques in food analysis—a review. Food Chem. 2019;273:3–8.
Furman OS, Teel AL, Watts RJ. Mechanism of base activation of persulfate. Environ Sci Technol. 2010;44:6423–8.
Georgakopoulos P, Zachari R, Mataragas M, Athanasopoulos P, Drosinos EH, Skandamis PN. Optimisation of octadecyl (C18) sorbent amount in QuEChERS analytical method for the accurate organophosphorus pesticide residues determination in low-fatty baby foods with response surface methodology. Food Chem. 2011;128:536–42.
Ghiasi A, Malekpour A, Mahpishanian S. Metal–organic framework MIL101 (Cr)-NH2 functionalized magnetic graphene oxide for ultrasonic-assisted magnetic solid phase extraction of neonicotinoid insecticides from fruit and water samples. Talanta. 2020;217:121120.
Ghouas H, Haddou B, Kameche M, Canselier J-P, Gourdon C. Removal of tannic acid from aqueous solution by cloud point extraction and investigation of surfactant regeneration by microemulsion extraction. J Surfactants Deterg. 2016;19:57–66.
Giakisikli G, Anthemidis AN. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta. 2013;789:1–16.
Guo H, Zhou X, Zhang Y, Yao Q, Qian Y, Chu H, Chen J. Carbamazepine degradation by heterogeneous activation of peroxymonosulfate with lanthanum cobaltite perovskite: performance, mechanism and toxicity. J Environ Sci. 2020;91:10–21.
Hammad M, Hardt S, Mues B, Salamon S, Landers J, Slabu I, Wende H, Schulz C, Wiggers H. Gas-phase synthesis of iron oxide nanoparticles for improved magnetic hyperthermia performance. J Alloys Compd. 2020;824:153.
Hasan M, Rashid MM, Hossain MM, Al Mesfer MK, Arshad M, Danish M, Lee M, El Jery A, Kumar N. Fabrication of polyaniline/activated carbon composite and its testing for methyl orange removal: optimization, equilibrium, isotherm and kinetic study. Polym Test. 2019;77:105.
Ike IA, Linden KG, Orbell JD, Duke M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem Eng J. 2018;338:651–69.
Javadi T, Farajmand B, Yaftian MR, Zamani A. Homogenizer assisted dispersive liquid-phase microextraction for the extraction-enrichment of phenols from aqueous samples and determination by gas chromatography. J Chromatogr A. 2020;1614:460733.
Jiang R, Xu J, Lin W, Wen S, Zhu F, Luan T, Ouyang G. Investigation of the kinetic process of solid phase microextraction in complex sample. Anal Chim Acta. 2015;900:111–6.
Jovanov P, Guzsvány V, Franko M, Lazić S, Sakač M, Šarić B, Banjac V. Multi-residue method for determination of selected neonicotinoid insecticides in honey using optimized dispersive liquid–liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta. 2013;111:125–33.
Jovanov P, Guzsvány V, Franko M, Lazić S, Sakač M, Milovanović I, Nedeljković N. Development of multiresidue DLLME and QuEChERS based LC–MS/MS method for determination of selected neonicotinoid insecticides in honey liqueur. Food Res Int. 2014;55:11–9.
Kachangoon R, Vichapong J, Santaladchaiyakit Y, Srijaranai S. Cloud-point extraction coupled to in-situ metathesis reaction of deep eutectic solvents for preconcentration and liquid chromatographic analysis of neonicotinoid insecticide residues in water, soil and urine samples. Microchem J. 2020;152:104377.
Komendova R. Recent advances in the preconcentration and determination of platinum group metals in environmental and biological samples. TrAC Trends Anal Chem. 2020;122:115708.
Li C, Huang J, Wang K, Chen Z, Liu Q. Optimization of processing parameters of laser skin welding in vitro combining the response surface methodology with NSGA- II. Infrared Phys Technol. 2019;103:103067.
Mahai G, Wan Y, **a W, Yang S, He Z, Xu S. Neonicotinoid insecticides in surface water from the central Yangtze River, China. Chemosphere. 2019;229:452–60.
Mashao G, Ramohlola KE, Mdluli SB, Monama GR, Hato MJ, Makgopa K, Molapo KM, Ramoroka ME, Iwuoha EI, Modibane KD. Zinc-based zeolitic benzimidazolate framework/polyaniline nanocomposite for electrochemical sensing of hydrogen gas. Mater Chem Phys. 2019;230:287–98.
Mashile PP, Mpupa A, Nomngongo PN. Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: kinetics and isotherms. Toxicon. 2018a;145:25–31.
Mashile GP, Mpupa A, Dimpe MK, Nomngongo PN. Magnetic activated carbon@ iron oxide@manganese oxide composite as an adsorbent for preconcentration of microcystin—LR in surface water, tap water, water and wastewater. Environ Nanotechnol Monit Manag. 2018b;10:199–205.
Mehdinia A, Aziz-Zanjani MO. Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction. TrAC Trends Anal Chem. 2013;51:13–22.
Mogolodi Dimpe K, Nomngongo PN. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J Pharm Anal. 2019;9:117–26.
Mpupa A, Mashile GP, Nomngongo PN. Ultrasound-assisted dispersive solid phase nanoextraction of selected personal care products in wastewater followed by their determination using high performance liquid chromatography-diode array detector. J Hazard Mater. 2019;370:33–41.
Mui EL, Cheung W, Valix M, McKay G. Mesoporous activated carbon from waste tyre rubber for dye removal from effluents. Microporous Mesoporous Mater. 2010;130:287–94.
Munonde TS. Preparation of modified magnetic nanocomposites and their application for preconcentration and speciation of selected trace elements in river water samples. Johannesburg: University of Johannesburg; 2017.
Naushad M, Alqadami AA, Al-Kahtani AA, Ahamad T, Awual MR, Tatarchuk T. Adsorption of textile dye using para-aminobenzoic acid modified activated carbon: kinetic and equilibrium studies. J Mol Liq. 2019;296:112075.
Njoku V, Foo K, Asif M, Hameed B. Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng J. 2014;250:198–204.
Peyton GR. The free-radical chemistry of persulfate-based total organic carbon analyzers. Mar Chem. 1993;41:91–103.
Pietrzak D, Kania J, Kmiecik E, Malina G, Wątor K. Fate of selected neonicotinoid insecticides in soil–water systems: current state of the art and knowledge gaps. Chemosphere. 2020;255:126981.
Ramohlola KE, Masikini M, Mdluli SB, Monama GR, Hato MJ, Molapo KM, Iwuoha EI, Modibane KD. Electrocatalytic hydrogen production properties of poly (3-aminobenzoic acid) doped with metal organic frameworks. Int J Electrochem Sci. 2017a;12:4392–405.
Ramohlola KE, Masikini M, Mdluli SB, Monama GR, Hato MJ, Molapo KM, Iwuoha EI, Modibane KD. Electrocatalytic hydrogen evolution reaction of metal organic frameworks decorated with poly (3-aminobenzoic acid). Electrochim Acta. 2017b;246:1174–82.
Rodríguez R, Avivar J, Leal LO, Cerdà V, Ferrer L. Strategies for automating solid-phase extraction and liquid–liquid extraction in radiochemical analysis. TrAC Trends Anal Chem. 2016;76:145–52.
Sadeghi MM, Rad AS, Ardjmand M, Mirabi A. Preparation of magnetic nanocomposite based on polyaniline/Fe3O4 towards removal of lead (II) ions from real samples. Synth Met. 2018;245:1–9.
Sánchez-Bayo F, Hyne RV. Detection and analysis of neonicotinoids in river waters—development of a passive sampler for three commonly used insecticides. Chemosphere. 2014;99:143–51.
Shabanian M, Khoobi M, Hemati F, Khonakdar HA, Wagenknecht U, Shafiee A. New PLA/PEI-functionalized Fe3O4 nanocomposite: preparation and characterization. J Ind Eng Chem. 2015;24:211–8.
Song X, **e J, Zhang M, Zhang Y, Li J, Huang Q, He L. Simultaneous determination of eight cyclopolypeptide antibiotics in feed by high performance liquid chromatography coupled with evaporation light scattering detection. J Chromatogr B. 2018;1076:103–9.
Spietelun A, Marcinkowski Ł, de la Guardia M, Namieśnik J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J Chromatogr A. 2013;1321:1–13.
Tarhan T, Ulu A, Sariçam M, Çulha M, Ates B. Maltose functionalized magnetic core/shell Fe3O4@Au nanoparticles for an efficient l-asparaginase immobilization. Int J Biol Macromol. 2020;142:443–51.
Treder N, Maliszewska O, Olędzka I, Kowalski P, Miękus N, Bączek T, Bień E, Krawczyk MA, Adamkiewicz-Drożynska E, Plenis A. Development and validation of a high-performance liquid chromatographic method with a fluorescence detector for the analysis of epirubicin in human urine and plasma, and its application in drug monitoring. J Chromatogr B. 2020;1136:121910.
Valasques GS, dos Santos AMP, de Souza VS, Teixeira LSG, Alves JPS, Souza MJ, dos Santos WPC, Bezerra MA. Multivariate optimization for the determination of cadmium and lead in crude palm oil by graphite furnace absorption atomic spectrometry after extraction induced by emulsion breaking. Microchem J. 2019;153:104.
Vichapong J, Burakham R, Srijaranai S. Vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction with solidification of floating organic droplet combined with HPLC for the determination of neonicotinoid pesticides. Talanta. 2013;117:221–8.
Vichapong J, Burakham R, Srijaranai S. In-coupled syringe assisted octanol–water partition microextraction coupled with high-performance liquid chromatography for simultaneous determination of neonicotinoid insecticide residues in honey. Talanta. 2015;139:21–6.
Vu HC, Dwivedi AD, Le TT, Seo S-H, Kim E-J, Chang Y-S. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr (VI) and As (V) from aqueous solutions: role of crosslinking metal cations in pH control. Chem Eng J. 2017;307:220–9.
Wang W, Li Y, Wu Q, Wang C, Zang X, Wang Z. Extraction of neonicotinoid insecticides from environmental water samples with magnetic graphene nanoparticles as adsorbent followed by determination with HPLC. Anal Methods. 2012;4:766–72.
Wang D, Chen Z, Zhou Z, Wang D, Yu J, Gao S. Catalytic upgrading of volatiles from coal pyrolysis over sulfated carbon-based catalysts derived from waste red oil. Fuel Process Technol. 2019;189:98–109.
Yamamoto I, Casida JE. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo: Springer; 1999.
Yáñez KP, Martín MT, Bernal JL, Nozal MJ, Bernal J. Trace analysis of seven neonicotinoid insecticides in bee pollen by solid–liquid extraction and liquid chromatography coupled to electrospray ionization mass spectrometry. Food Anal Methods. 2014;7:490–9.
Yi X, Zhang C, Liu H, Wu R, Tian D, Ruan J, Zhang T, Huang M, Ying G. Occurrence and distribution of neonicotinoid insecticides in surface water and sediment of the Guangzhou section of the Pearl River, South China. Environ Pollut. 2019;251:892–900.
Zare EN, Lakouraj MM, Ashna A. Synthesis of conductive poly (3-aminobenzoic acid) nanostructures with different shapes in acidic ionic liquids medium. J Mol Liq. 2018;271:514–21.
Zhang B-T, Zheng X, Li H-F, Lin J-M. Application of carbon-based nanomaterials in sample preparation: a review. Anal Chim Acta. 2013;784:1–17.
Zhang J, Li R, Ding G, Wang Y, Wang C. Sorptive removal of phenanthrene from water by magnetic carbon nanomaterials. J Mol Liq. 2019;293:111540.
Zhao Y, Gong X, Si X, Wei Z, Yang C, Zhang S, Zhang X. Coupling a solid phase microextraction (SPME) probe with ambient MS for rapid enrichment and detection of phosphopeptides in biological samples. Analyst. 2015;140:2599–602.
Zhao G, Zou J, Chen X, Liu L, Wang Y, Zhou S, Long X, Yu J, Jiao F. Iron-based catalysts for persulfate-based advanced oxidation process: microstructure, property and tailoring. Chem Eng J. 2020;421:127845.
Zhou Q, Ding Y, **ao J. Sensitive determination of thiamethoxam, imidacloprid and acetamiprid in environmental water samples with solid-phase extraction packed with multiwalled carbon nanotubes prior to high-performance liquid chromatography. Anal Bioanal Chem. 2006;385:1520–5.
Zou Y, Li W, Yang L, **ao F, An G, Wang Y, Wang D. Activation of peroxymonosulfate by sp2-hybridized microalgae-derived carbon for ciprofloxacin degradation: importance of pyrolysis temperature. Chem Eng J. 2019;370:1286–97.
Acknowledgements
The authors further appreciate the University of Johannesburg for allowing us to utilize their laboratory facilities to make this study possible.
Funding
The authors would like to give thanks to the National Nanoscience Postgraduate Teaching and Training Platform (NNPTTP) for financial support and National Research Foundation (NRF, South Africa, Grant No. 99270&91230).
Author information
Authors and Affiliations
Contributions
NJW involved in conceptualization, conducting experiments, investigation, writing–original draft, writing–review and editing, experimental design, methodology and interpretation of data. AM involved in conceptualization, writing–review and editing, experimental design, methodology and interpretation of data; SKS involved in conducting experiments, writing–review and editing and interpretation of data. PNN involved in conceptualization, investigation, project administration software, experimental design, writing–review and editing and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests associated with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1. List of runs obtained from two-level 25-1 fractional factorial design and their responses for screening. Table S2. List of runs obtained from small central composite design (SCCD) and their responses for second optimization. Figure S1. Desirability profiles of (A) ACT, (B) IMD and (C) CLD. Figure S2. Percentage recovery vs number of cycles for (A) ACT, (B) IMD, (C) TCL and (D) CLD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waleng, N.J., Selahle, S.K., Mpupa, A. et al. Development of dispersive solid-phase microextraction coupled with high-pressure liquid chromatography for the preconcentration and determination of the selected neonicotinoid insecticides. J Anal Sci Technol 13, 4 (2022). https://doi.org/10.1186/s40543-021-00311-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-021-00311-4